


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 24-9-2020
التاريخ: 31-10-2019
التاريخ: 2023-08-08
التاريخ: 2023-08-28
|
Ionic or polar reactions of alkyl halides rarely are observed in the vapor phase because the energy required to dissociate a carbon-halogen bond heterolytically is almost prohibitively high. For example, while the heat of dissociation of chloromethane to a methyl radical and a chlorine atom is 84kcal mol−1 (Table 4-6), dissociation to a methyl cation and a chloride ion requires about 227kcal mol−1:
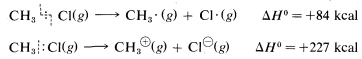
However, the heat of ionic dissociation of methyl chloride in aqueous solution is estimated to be 63kcal, and while this reaction is still substantially endothermic, it requires about 227−63=164kcal less energy than in the gas phase:
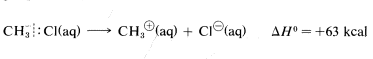
The reason is that ions are much more stable in water than in the gas phase; for example, the transfer of a chloride ion from the gas to water is exothermic by −85kcal−1. The ΔH0 value for the corresponding transfer of a methyl cation, CH3⊕, is not known with certainty, but is about −80kcal. These ionic solvation energies are clearly large. In contrast, the ΔH0 for solution of methyl chloride in water is small (about 1kcal). We can use these data to calculate the heat of ionic dissociation of chloromethane in water:
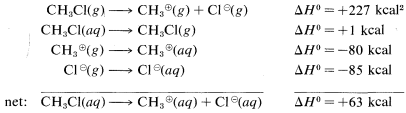
Thermochemical data for the solvation of ions as used in the preceding calculations are difficult to measure and even to estimate. Therefore this kind of calculation of ΔH0 for ionic reactions involving organic molecules in solution usually cannot be made. As a result, we have considerably fewer possibilities to assess the thermodynamic feasibility of the individual steps of polar reactions in solution than we do of vapor-phase radical processes. Bond energies are not of much use in predicting or explaining reactivity in ionic reactions unless we have some information that can be used to translate gas-phase ΔH0 values to solution ΔH0 values.
Calculated from the following data:
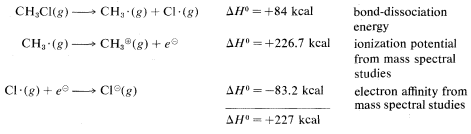



|
|
|
|
دور في الحماية من السرطان.. يجب تناول لبن الزبادي يوميا
|
|
|
|
|
|
|
العلماء الروس يطورون مسيرة لمراقبة حرائق الغابات
|
|
|
|
|
|
|
انطلاق الجلسة البحثية الرابعة لمؤتمر العميد العلمي العالمي السابع
|
|
|