
Experimental procedures
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
ص178-179
الجزء والصفحة:
ص178-179
 2025-11-15
2025-11-15
 28
28
Experimental procedures
Detecting a phase change is not always as simple as seeing water boil in a kettle, so special techniques have been developed. Two techniques are thermal analysis, which takes advantage of the effect of the enthalpy change during a first-order transition (Section 4.7), and differential scanning calorimetry (see Impact I2.1). They are useful for solid–solid transitions, where simple visual inspection of the sample may be inadequate. In thermal analysis, a sample is allowed to cool and its temperature is monitored. At a first-order transition, heat is evolved and the cooling stops until the transition is complete. The cooling curve along the is obarcde in Fig. 6.3 therefore has the shape shown in Fig. 6.4. The transition temperature is obvious, and is used to mark point d on the phase diagram. Modern work on phase transitions often deals with systems at very high pressures, and more sophisticated detection procedures must be adopted. Some of the highest pressures currently attainable are produced in adiamond-anvil cell like that illustrated in Fig. 6.5. The sample is placed in a minute cavity between two gem-quality diamonds, and then pressure is exerted simply by turning the screw. The advance in design this represents is quite remarkable for, with a turn of the screw, pressures of up to about 1 Mbar can be reached that a few years ago could not be reached with equipment weighing tons. The pressure is monitored spectroscopically by observing the shift of spectral lines in small pieces of ruby added to the sample, and the properties of the sample itself are observed optically through the diamond anvils. One application of the technique is to study the transition of covalent solids to metallic solids. Iodine, I2, for instance, becomes metallic at around 200 kbar and makes a transition to a monatomic metallic
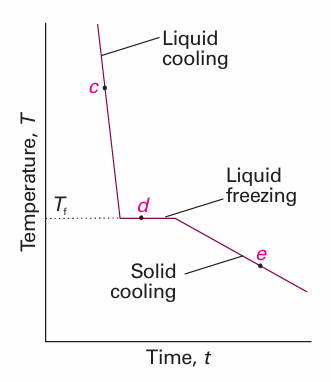
Fig. 6.4 The cooling curve for the is obarcde in Fig. 6.3. The halt marked d corresponds to the pause in the fall of temperature while the first-order exothermic transition (freezing) occurs. This pause enables Tf to be located even if the transition cannot be observed visually.
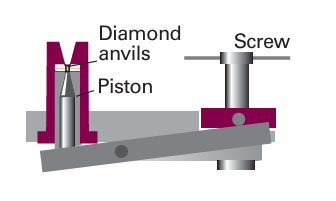
Fig. 6.5 Ultrahigh pressures (up to about 2 Mbar) can be achieved using a diamond anvil. The sample, together with a ruby for pressure measurement and a drop of liquid for pressure transmission, are placed between two gem-quality diamonds. The principle of its action is like that of a nutcracker: the pressure is exerted by turning the screw by hand.
solid at around 210 kbar. Studies such as these are relevant to the structure of material deep inside the Earth (at the centre of the Earth the pressure is around 5 Mbar) and in the interiors of the giant planets, where even hydrogen may be metallic.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


