
Activities in terms of molalities
 المؤلف:
Peter Atkins، Julio de Paula
المؤلف:
Peter Atkins، Julio de Paula
 المصدر:
ATKINS PHYSICAL CHEMISTRY
المصدر:
ATKINS PHYSICAL CHEMISTRY
 الجزء والصفحة:
161
الجزء والصفحة:
161
 2025-11-15
2025-11-15
 29
29
Activities in terms of molalities
The selection of a standard state is entirely arbitrary, so we are free to choose one that best suits our purpose and the description of the composition of the system. In chemistry, compositions are often expressed as molalities, b, in place of mole fractions. It therefore proves convenient to write , µB =µBo + RT ln bB .
where µo has a different value from the standard values introduced earlier. According to this definition, the chemical potential of the solute has its standard value µ7 when the molality of B is equal to b7 (that is, at 1 mol kg−1). Note that as bB → 0, µB →−∞; that is, as the solution becomes diluted, so the solute becomes increasingly stabilized. The practical consequence of this result is that it is very difficult to remove the last traces of a solute from a solution.
Now, as before, we incorporate deviations from ideality by introducing a dimensionless activity aB, a dimensionless activity coefficient γB, and writing
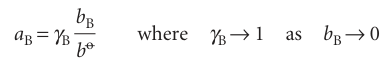
at all temperatures and pressures. The standard state remains unchanged in this last stage and, as before, all the deviations from ideality are captured in the activity coefficient γB. We then arrive at the following succinct expression for the chemical potential of a real solute at any molality: µ=µo+ RT ln a .
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


