


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 24-3-2016
Date: 23-3-2016
Date: 23-3-2016
|
Antibiotic Pharmacodynamics
The term antibiotic pharmacodynamics refers to the manner in which antibiotics interact with their target organisms to exert their effects: Does the antibiotic kill the organism or just prevent its growth? Is it better to give a high dose of antibiotics all at once or to achieve lower concentrations for a longer time? Clinicians increasingly recognize such considerations as important in maximizing the success of therapy, especially for difficult-to-treat infections or in immunocompromised patients.
Susceptibility Testing
Typically, one judges the susceptibility of a particular organism to an antibiotic based on the minimum inhibitory concentration (MIC) for the organism-antibiotic combination. The microbiology laboratory determines the MIC by mixing a standard concen-tration of the organism that the patient has grown with increasing concentrations of the antibiotic in a broth solution. Classically this was done in test tubes (Figure 1 ), but today it is done more commonly on microdilution plates. The mixture is incubated for about a day, and the laboratory technician examines the tubes or plates (with the naked eye or with a computer) for signs of cloudiness, indicating growth of the organism. The mixture with the
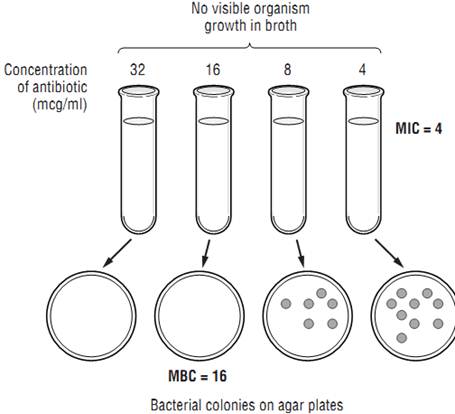
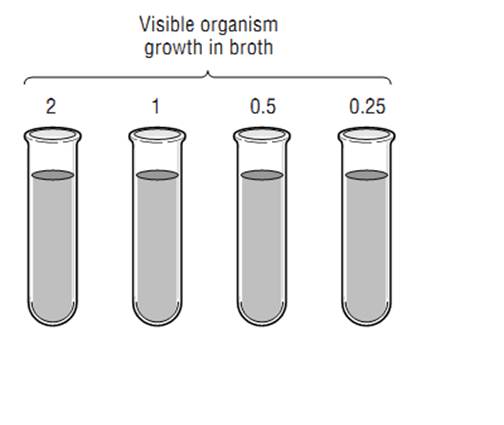
Figure 1: Susceptibility Testing of Antibiotics
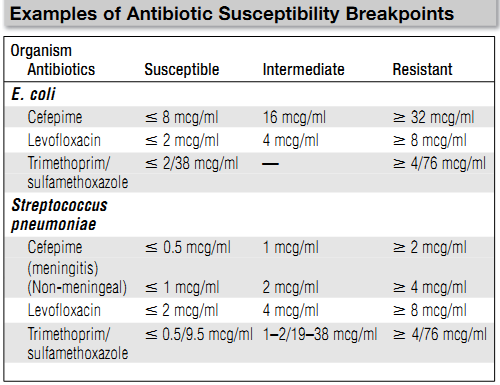
lowest concentration of antibiotic where there is no visible growth is deemed to be the MIC. For each organism-antibiotic pair there is a particular cutoff MIC that is considered susceptible. This particular MIC is called the breakpoint. Tables 1 provides examples of how breakpoints differ for various organ-ism/pathogen combinations and even based on the site of infection. Note that just because an antibiotic has the lowest MIC for a pathogen, that does not mean it is the best choice—different antibiotics achieve different concentrations in the body in different places. Thus, antibiotic MICs for a single organism generally should not be compared across different drugs in selecting therapy. Finally, be aware that other methods of susceptibility testing exist, including disk diffusion and E-tests, but that broth dilution methods are generally considered the gold standard.
Table 1
Static Versus Cidal
At the MIC the antibiotic is inhibiting growth, but it may or may not actually be killing the organism. Antibiotics that inhibit growth of the organism without killing it are termed bacteriostatic (or fungistatic in the case of fungi). If antibiotics are removed, the organisms can begin growing again. However, bacteriostatic antibiotics are usually successful in treating infections because they allow the patient’s immune system to catch up and kill off the organisms. Other antibiotics are considered bactericidal; their action kills the organisms without any help from the immune system.
For most infections, outcomes using appropriate bacteriostatic versus bactericidal drugs are similar; however, for certain infections bactericidal drugs are preferred. Such infections include endocarditis, meningitis, infections in neutropenic patients, and possibly osteomyelitis. The immune system may not be as effective in fighting these infections because of the anatomic location or- the immunosuppression of the patient. Bactericidal activity is determined by taking a sample of the broth at various concentrations and below and spreading the broth on agar plates (Figure 1). The bacterial colonies on the plates are counted, and the concentration corresponding to a 99.9% reduction in the original bacterial inoculum is considered to be the minimum bactericidal concentration (MBC). When the MBC is 4 times or less the MIC, the drug is considered to be bactericidal; if the MBC/MIC ratio is greater than 4, it is considered bacteriostatic. MIC testing is standard in most laboratories; MBC testing is more difficult and is not commonly done. Table 2 lists drugs and indicates whether they are generally considered bacteriostatic or bactericidal; however, it should be noted that this activity can vary based on the pathogen being treated, the achievable dose, and the growth phase of the organism.
Table 2

Pharmacokinetic/Pharmacodynamic Relationships
Besides differing in whether they kill microorganisms or merely inhibit their growth, antibiotics also differ in how they manifest their effects over time. Careful studies have revealed that for certain antibiotics, activity against microorganisms correlates with the duration of time that the concentration of the drug remains above the MIC (time-dependent activity). For other antibiotics, antibacterial activity correlates not with the time above the MIC but with the ratio of the peak concentration of the drug to the MIC (concentration-dependent or time-independent activity). For some antibiotics, the best predictor of activity is the ratio of the area under the concentration-time curve to the MIC. Figure2 illustrates these pharmacokinetic/pharmacodynamic (PK/PD) parameters schematically, and Table 2 shows which parameter is most predictive of efficacy for antibiotic classes. The practical implications of these findings are in the design of antibiotic dosing schedules: aminoglycosides are now frequently given as a single large dose daily to leverage the concentration-dependent activity, while some clinicians are administering beta-lactam drugs such as ceftazidime as continuous or prolonged infusions because of their time-dependent activity. As target values for these parameters that predict efficacy are found, there may be an increase in the individualization of dosing of antibiotics to achieve these target values.
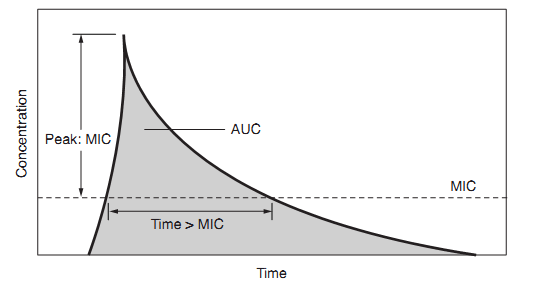
Figure 2: Pharmacokinetic/Pharmacodynamic Relationships
References
Gallagher ,J.C. and MacDougall ,c. (2012). Antibiotics Simplified. Second Edition. Jones & Bartlett Learning, LLC.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|