
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 10-5-2019
Date: 10-7-2018
Date: 10-10-2019
|
Tertiary alcohols are not oxidized by acidified sodium or potassium dichromate(VI) solution - there is no reaction whatsoever. If you look at what is happening with primary and secondary alcohols, you will see that the oxidizing agent is removing the hydrogen from the -OH group, and a hydrogen from the carbon atom attached to the -OH. Tertiary alcohols don't have a hydrogen atom attached to that carbon.
You need to be able to remove those two particular hydrogen atoms in order to set up the carbon-oxygen double bond.
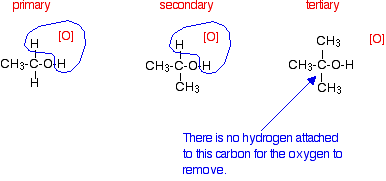



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ضمن أسبوع الإرشاد النفسي.. جامعة العميد تُقيم أنشطةً ثقافية وتطويرية لطلبتها
|
|
|