


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 4-10-2020
Date: 27-8-2018
Date: 21-7-2016
|
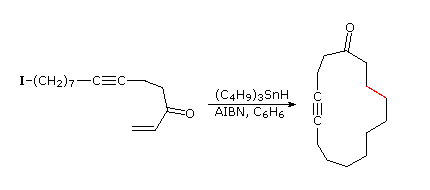
If a radical is joined to a double bond by a chain of three or more carbons intramolecular addition generates a ring. The regioselectivity of such additions is governed more by stereoelectronic factors than by substituents on the double bond. In the first two examples shown below, double bond substitution would favor formation of a six-membered ring, but five-membered ring formation by way of a 1º-cyclized radical dominates the products. Likewise, in reaction 3 a six-membered ring is formed preferentially over an alternative seven-membered ring. In each of the examples the ring forming bond is colored red. . Note that these reactions tolerate a wide variety of functional groups.
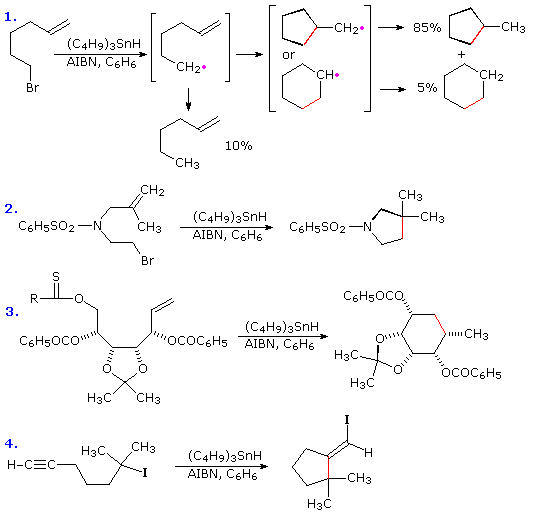
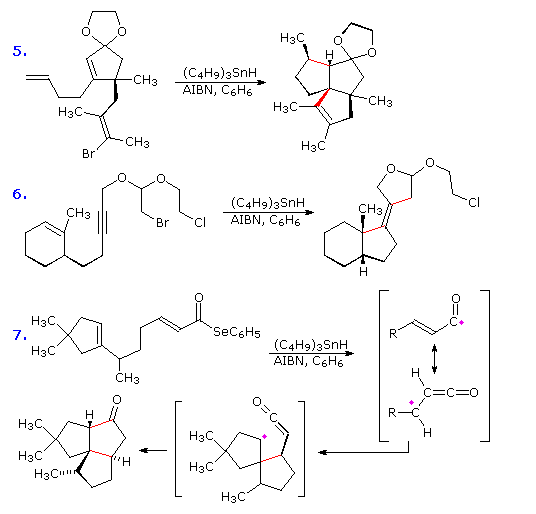
The stereoelectronic factor in this reaction is defined by the preferred mode of approach of a radical as it bonds to the pi-electron system of an alkene function. As shown in the following diagram, this is at an angle nearly 20º off the perpendicular to the plane of the double bond. Because of this requirement, many cyclizations to moderately sized rings proceed by radical attack at the nearest carbon of the double bond, regardless of substitution. Bonding to the distal carbon is constrained by the structure of the connecting chain. Of course, if the carbon chain tethering the radical site to the double bond is long enough, bonding to either of the double bond carbons accommodates the stereoelectronic factor, and the product is again determined by substitution.
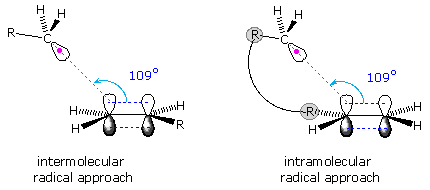



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
جمعية العميد تعقد اجتماعًا لمناقشة المشاريع العلمية والبحثية
|
|
|