


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية| Membrane filtration method APHA 2001 and Plate count method APHA 2001 for osmophilic yeasts in foods |
|
|
|
Read More
Date: 17-11-2015
Date: 17-11-2015
Date: 22-3-2016
|
Membrane filtration method APHA 2001 and Plate count method APHA 2001 for osmophilic yeasts in foods
Method of the American Public Health Association (APHA), as described in the 4th Edition of the Compendium of Methods for the Microbiological Examination of Foods (Baross, 2001).
1- Material required for analysis
Membrane filter method
• Sterile distilled water or equivalent
• Malt Extract Yeast Extract 40% Glucose (MY40G) Agar
• Membrane filters 0.80 μm pore size
• Sterile filtration apparatus
• Vacuum pump
• Laboratory incubator set to 30±1°C
Plate count method
• Butterfield’s Phosphate Buffer with 40% Glucose
(w/w)
• Malt Extract Yeast Extract 40% Glucose (MY40G) Agar
• Laboratory incubator set to 30±1°C
2- Procedure
A general flowchart for the enumeration of osmophilic yeasts using the membrane filtration method or the plate count method APHA 2001 is shown in Figure 1.
The Compendium presents two procedures for the enumeration of osmophilic yeasts, one using the membrane filtration technique and the other using the direct plating technique.
a) Membrane filtration method: This method is recommended for filterable non-spoiled samples or for the rinsing water of processing lines, in which the population of osmophilic yeasts may be very low, but even so still be potentially capable of causing spoilage. It is important to consider that dilution of the sample with sterile distilled water, though still needed to make filtration possible, may subject the osmophilic yeasts contained in the sample to osmotic shock. The resulting possible reduction in the number of CFU obtained must always be taken into account when interpreting the results.
Procedure: Weigh 25 g of the sample and add 25–50 ml of sterile, reagent grade water, homogenizing well (in the specific case of samples of rinsing water from processing lines there is no need for dilution). Filter through a filter membrane with a pore size of 0.80 μm, graph side facing up. Before the membrane dries, wash the walls of the cup of the filtration system with 100 ml sterile distilled water, filtering the whole volume of water to wash the membrane. Turn off the vacuum pump before the membrane starts to dry excessively. Transfer the membrane to a plate containing Malt Extract Yeast Extract 40% Glucose (MY40G) Agar, placing it onto the surface of the culture medium, graph side up. Incubate the plate at 30ºC for five to seven days.
Note a.1) It is recommended that liquid sugar samples be filtered preferably using metal filter holders, since porous filter holders are difficult to clean and sanitize after filtering this kind of product.
b) Plate count method: This method is recommended for samples that are not suited for filtration, such as concentrated juices and fruit syrups. It is also the most indicated for samples with a high population of osmophilic yeasts, because it allows inoculation of dilutions.
Procedure: Weigh 25 g of the sample and add 225 ml of Butterfield’s Phosphate Buffer supplemented with 40% glucose (weight/weight) (dilution 10−1). In the case of analysis of sugar, prepare the diluent without the addition of glucose and add 40% of the sample itself (or an equivalent weight, in the case of liquid sugar) to the diluent, considering this dilution as the undiluted sample. Prepare the subsequent dilutions, using the same diluent.
Inoculate 1 ml of the undiluted sample, 1 ml of the 10−1 dilution and 1 ml of the 10−2 dilution in sterile Petri dishes and add approximately 20 ml of Malt Extract Yeast Extract 40% Glucose (MY40G) Agar. Select higher dilutions in the case of spoiled samples, in which the expected counts are higher. Incubate the plates at 30ºC for five to seven days and count the colonies with the aid of a magnifying glass of a colony counter. Only osmophilic yeasts will be able to develop in MY40G agar forming very small colonies after this incubation period, since the growth rate of these microorganisms is low as a result of the water activity conditions of the medium .
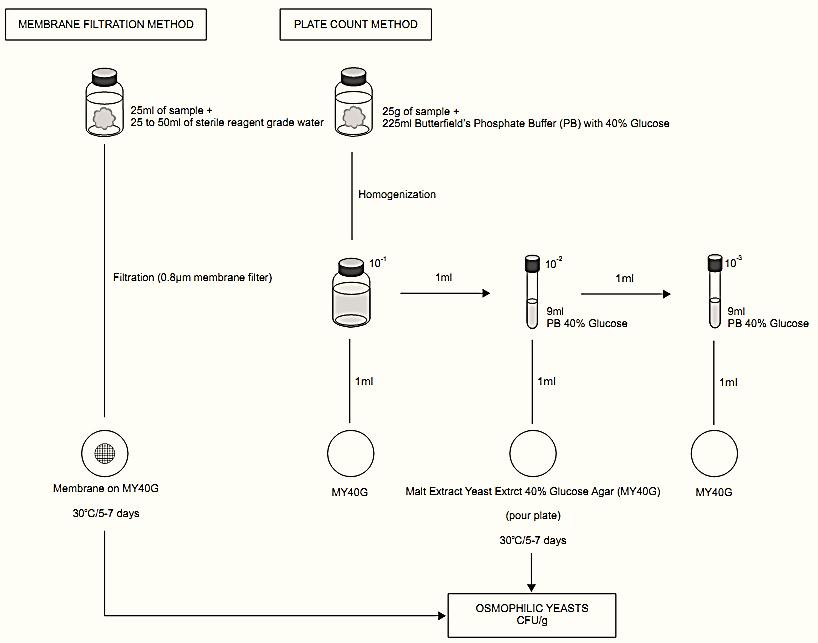
Figure 1 Scheme of analysis for the enumeration of osmophilic yeasts using the membrane filtration or the plate count method APHA 2001 (Baross, 2001).
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil.
Baross, J.A. (2001) Halophilic and osmophilic microorganisms. In: Downes, F.P. & Ito, K. (eds). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 17, pp. 187–199.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|