


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 6-1-2019
التاريخ: 15-1-2019
التاريخ: 24-12-2018
التاريخ: 1-4-2019
|
Physical properties of d-block metals
Nearly all the d-block metals are hard, ductile and malleable, with high electrical and thermal conductivities. With the exceptions of Mn, Zn, Cd and Hg, at room temperature, the metals possess one of the typical metal structures. The metallic radii (rmetal) for 12-coordination (Figure 1.) are much smaller that those of the s-block metals of comparable atomic number; Figure 1 also illustrates that values of rmetal:
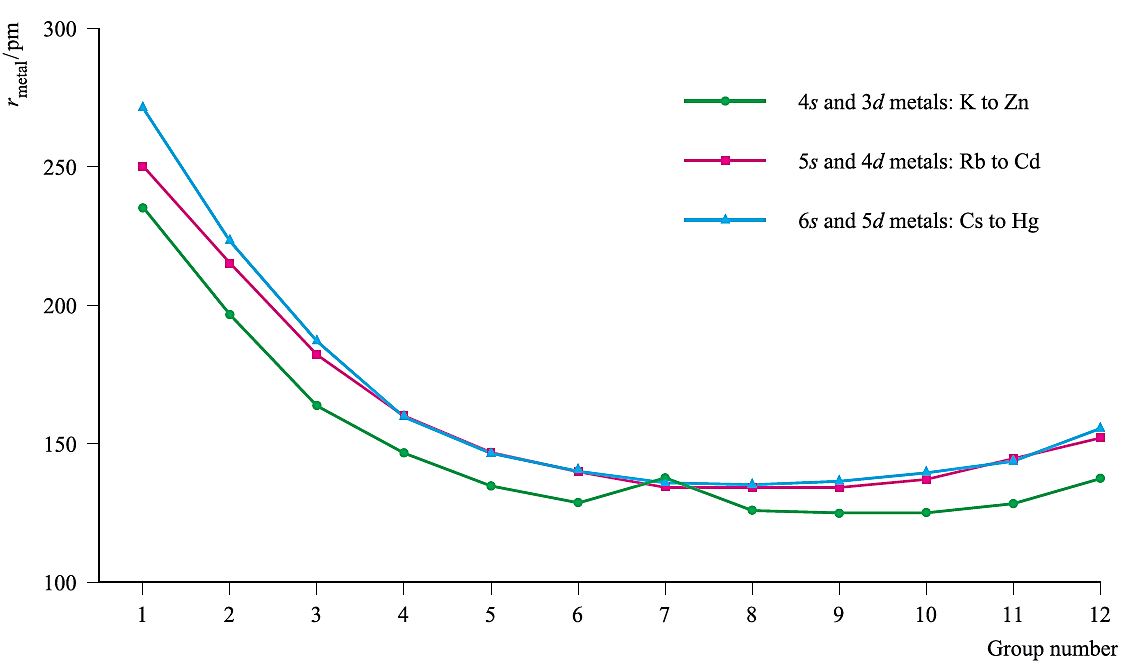
Fig. 1. Trends in metallic radii (rmetal) across the three rows of s- and d-block metals K to Zn, Rb to Cd, and Cs to Hg.
This last observation is due to the so-called lanthanoid contraction. Metals of the d-block are (with the exception of the group 12 metals) much harder and less volatile than those of the sblock.
The trends in enthalpies of atomization are shown in Figure 2. Metals in the second and third rows generally possess higher enthalpies of atomization than the corresponding elements in the first row; this is a substantial factor in accounting for the far greater occurrence of metal–metal bonding in compounds of the heavier d-block metals compared with their first row congeners. In general, Figure 2 shows that metals in the centre of the d-block possess higher values of ΔaHo(298 K) than early or late metals. However, one must be careful in comparing metals with different structure types and this is particularly true of manganese. The first ionization energies (IE1) of the d-block metals in a given period are higher than those of the preceding s-block metals. Figure 3. shows that across each of the periods K to Kr, Rb to Xe, and Cs to Rn, the variation in values of IE1 is small across the d-block and far greater among the s- and p-block elements. Within each period, the overall trend for the d-block metals is for the ionization energies to increase, but many small variations occur. Chemical comparisons between metals from the sand d-blocks are complicated by the number of factors involved. Thus, all 3d metals have values of IE1 and IE2 larger than those of calcium, and all except zinc have higher values of ΔaHo (Figure 2); these factors make the metals less reactive than calcium. However, since all known M2+ ions of the 3d metals are smaller than Ca2+, lattice and solvation energy effects are more favourable for the 3d metal ions.
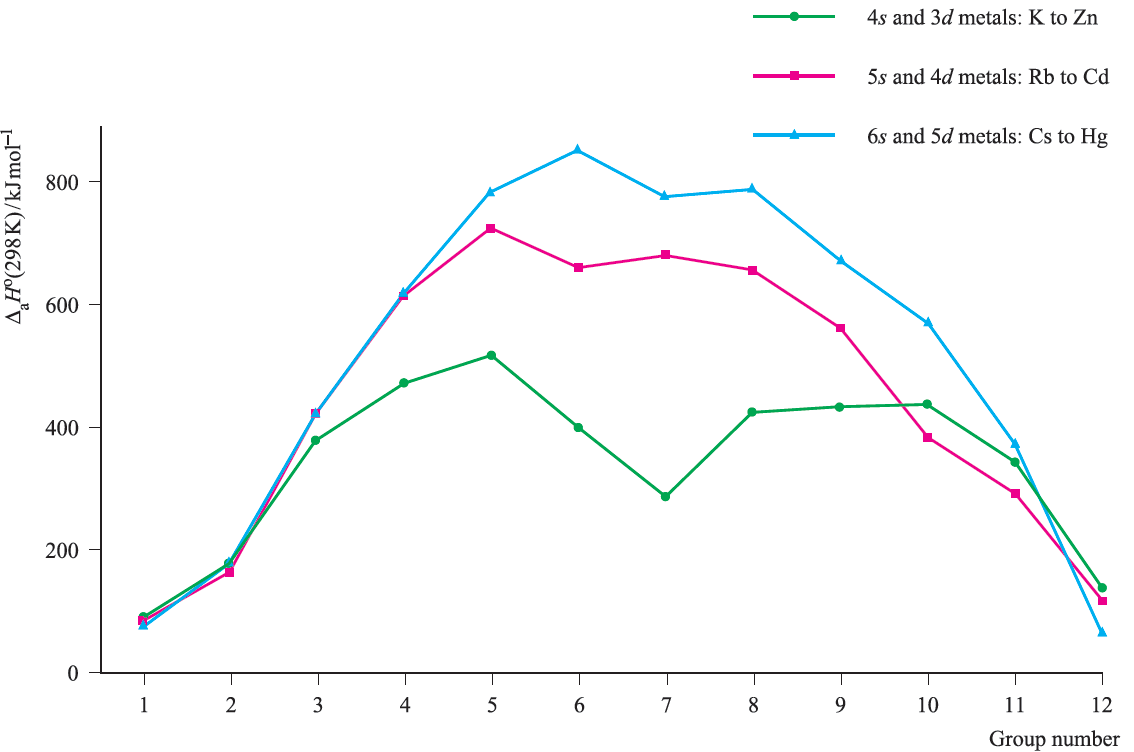
Fig.2. Trends in standard enthalpies of atomization, ΔaHo(298 K), across the three rows of s- and d-block metals K to Zn, Rb to Cd, and Cs to Hg.
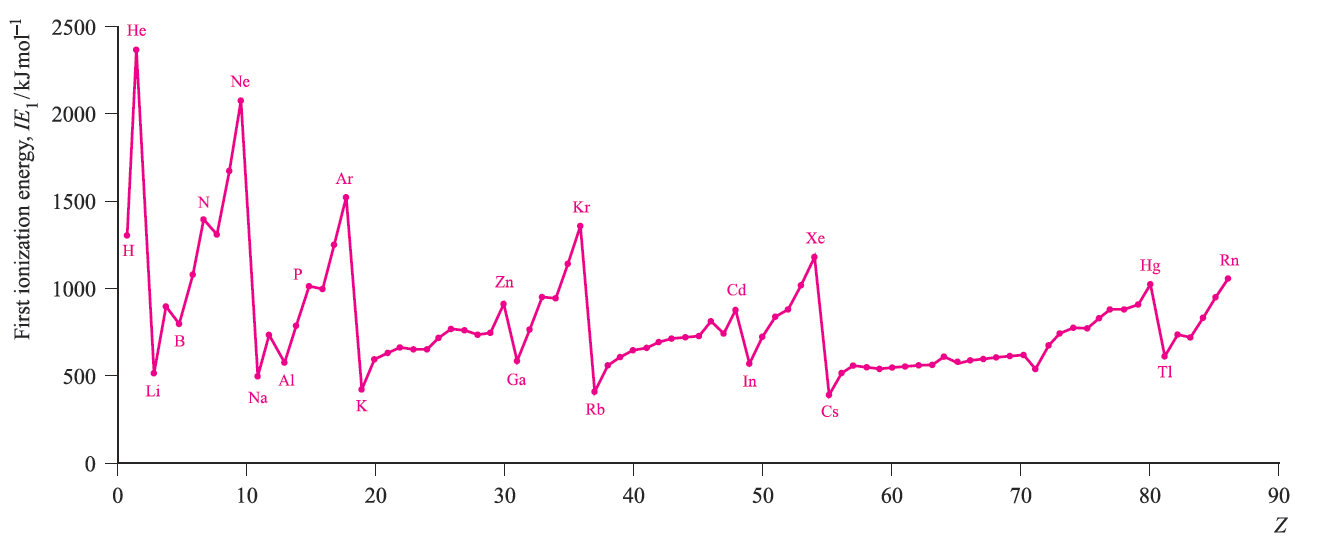
figure 3. The values of the first ionization energies of the elements up to Rn.
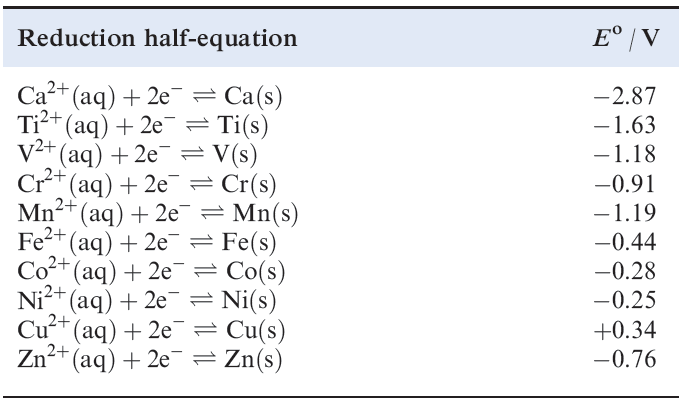
In practice, it turns out that, in the formation of species containing M2+ ions, all the 3d metals are thermodynamically less reactive than calcium, and this is consistent with the standard reduction potentials listed in Table 1.1 However, interpretation of observed chemistry based on these Eo data is not always straightforward, since the formation of a coherent surface film of metal oxide often renders a metal less reactive than expected . A few d-block metals are very powerful reducing agents, e.g. Eo for the Sc3+/Sc couple (_2.08 V) is more negative than that for Al3+/Al (_1.66 V).
Table 1.1 Standard reduction potentials (298 K) for some metals in the first long period; the concentration of each aqueous solution is 1 moldm-3.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|