


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-2-2016
Date: 12-2-2016
Date: 12-2-2016
|
NITRATION
Nitration is the insertion of a nitro group (-NO2) into an organic compound, usually through the agency of the reaction of a hydrocarbon with nitric acid. Concentrated sulfuric acid may be used as a catalyst.
ArH + HNO3 → ArNO2 + H2O
More than one hydrogen atom may be replaced, but replacement of each succeeding hydrogen atom represents a more difficult substitution. The nitrogen-bearing reactant may be:
1. Strong nitric acid
2. Mixed nitric and sulfuric acid
3. A nitrate plus sulfuric acid
4. Nitrogen pentoxide (N2O5)
5. A nitrate plus acetic acid
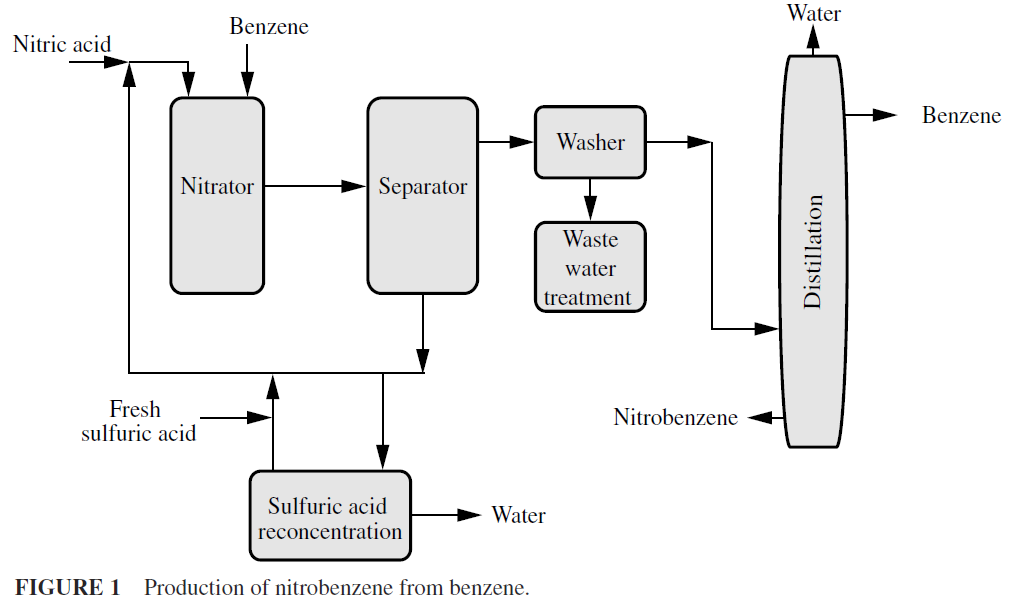
Both straight chain and ring-type carbon compounds can be nitrated; alkanes yield nitroparaffins. The process for the production of nitrobenzene from benzene involves the use of mixed acid (Fig. 1), but there are other useful nitrating agents, e.g., inorganic nitrates, oxides of nitrogen, nitric acid plus acetic anhydride, and nitric acid plus phosphoric acid. In fact, the presence of sulfuric acid in quantity is vital to the success of the nitration because it increases the solubility of the hydrocarbon in the reaction mix, thus speeding up the reaction, and promotes the ionization of the nitric acid to give the nitronium ion (NO2+), which is the nitrating species. Absorption of water by sulfuric acid favors the nitration reaction and shifts ther eaction equilibrium to the product.
Nitration offers a method of making unreactive paraffins into reactive substances without cracking. Because nitric acid and nitrogen oxides are strong oxidizing agents, oxidation always accompanies nitration. Aromatic nitration reactions have been important particularly for the manufacture of explosives. Nitrobenzene is probably the most important nitration product.
Certain esters of nitric acid (cellulose nitrate, glyceryl trinitrate) are often referred to as nitro compounds (nitrocellulose, nitroglycerin), but this terminology should be avoided. Vapor-phase nitration of paraffin hydrocarbons, particularly propane, can be brought about by uncatalyzed contact between a large excess of hydrocarbon and nitric acid vapor at around 400oC, followed by quenching. A multiplicity of nitrated and oxidized products results from nitrating propane; nitromethane, nitroethane, nitropropanes, and carbon dioxide all appear, but yields of useful products are fair. Materials of construction must be very oxidation-resistant and are usually of ceramic-lined steel. The nitroparaffins have found limited use as fuels for race cars, submarines, and model airplanes. Their reduction products, the amines, and other hydroxyl compounds resulting from aldol condensations have made a great many new aliphatic yntheses possible because of their ready reactivity.
Nitration reactions are carried out in closed vessels that are provided with an agitating mechanism and means for controlling the reaction temperature. The nitration vessels are usually constructed of cast iron and steel, but often acid-resistant alloys, particularly chrome-nickel steel alloys, are used. Plants may have large (several hundred gallon capacity) nitration vessels operating in a batch mode or small continuous units. The temperature is held at about 50oC, governed by the rate of feed of benzene. Reaction is rapid in well-stirred and continuous nitration vessels. The reaction products are decanted from the spent acid and are washed with dilute alkali. The spent acid is sent to some type of recovery system and yields of 98 percent can be anticipated.
Considerable heat evolution accompanies the nitration reaction, oxidation increases it, and the heat of dilution of the sulfuric acid increases it still further. Increased temperature favors dinitration arid oxidation, so the reaction must be cooled to keep it under control. Good heat transfer can be assured by the use of jackets, coils, and good agitation in the nitration vessel. Nitration vessels are usually made of stainless steel, although cast iron stands up well against mixed acid.
When temperature regulation is dependent solely on external jackets, a disproportional increase in nitration vessel capacity as compared with jacket surface occurs when the size of the machine is enlarged. Thus, if the volume is increased from 400 to 800 gallons, the heat-exchange area increases as the square and the volume as the cube of the expanded unit. To overcome this fault, internal cooling coils or tubes are introduced, which have proved satisfactory when installed on the basis of sound calculations that include the several thermal factors entering into this unit
process.
A way of providing an efficient agitation inside the nitration vessel is essential if local overheating is to be mitigated. Furthermore, the smoothness of the reaction depends on the dispersion of the reacting material as it comes in contact with the change in the nitration vessel so that a fairly uniform temperature is maintained throughout the vessel. Nitration vessels are usually equipped with one of three general types of agitating mechanism: (1) single or double impeller, (2) propeller or turbine, with cooling sleeve, and (3) outside tunnel circulation. The single-impeller agitator consists of one vertical shaft containing horizontal arms. The shaft may be placed off center in order to create rapid circulation past, or local turbulence at, the point of contact between the nitrating acid and the organic compound.
The double-impeller agitator consists of two vertical shafts rotating in opposite directions, and each shaft has a series of horizontal arms attached.
The lower blades have an upward thrust, whereas the upper ones repel the liquid downward. This conformation provides a reaction mix that is essentially homogeneous. The term sleeve-and-propeller agitation is usually applied when the nitration vessel is equipped with a vertical sleeve through which the charge is circulated by the action of a marine propeller or turbine. The sleeve is usually made of a solid bank of acid-resisting cooling coils through which cold water or brine is circulated at a calculated rate. In order to obtain the maximum efficiency with this type of nitration vessel, it is essential to maintain a rapid circulation of liquid upward or downward in the sleeves and past the coils.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مدرسة دار العلم.. صرح علميّ متميز في كربلاء لنشر علوم أهل البيت (عليهم السلام)
|
|
|