


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 20-9-2017
Date: 12-1-2020
Date: 12-1-2020
|
In a linear polymer such as polyethylene, rotations around carbon-carbon single bonds can allow the chains to bend or curl up in various ways, resulting in the spaghetti-like mixture of these different conformations we alluded to above. But if one of the hydrogen atoms is replaced by some other entity such as a methyl group, the relative orientations of the individual monomer units that make up a linear section of any carbon chain becomes an important characteristic of the polymer.
Cis-trans isomerism occurs because rotation around carbon-carbon double bonds is not possible — unlike the case for single bonds. Any pair of unlike substituents attached to the two carbons is permanently locked into being on the same side (cis) or opposite sides (trans) of the double bond.
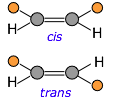
If the carbon chain contains double bonds, then cis-trans isomerism becomes possible, giving rise to two different possible configurations (known as diastereomers) at each unit of the chain. This seemingly small variable can profoundly affect the nature of the polymer. For example, the latex in natural rubber is made mostly of cis-polyisoprene, whereas the trans isomer (known as gutta percha latex) has very different (and generally inferior) properties.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|