


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 27-6-2017
Date: 31-1-2017
Date: 13-6-2019
|
All normal matter at temperatures above absolute zero emits electromagnetic radiation, which represents a conversion of a body's internal thermal energy into electromagnetic energy, and is therefore called thermal radiation. Conversely, all normal matter absorbs electromagnetic radiation to some degree. An object that absorbs ALL radiation falling on it, at all wavelengths, is called a blackbody. When a blackbody is at a uniform temperature, its emission has a characteristic frequency distribution that depends on the temperature. This emission is called blackbody radiation.
A room temperature blackbody appears black, as most of the energy it radiates is infra-red and cannot be perceived by the human eye. Because the human eye cannot perceive light waves at lower frequencies, a black body, viewed in the dark at the lowest just faintly visible temperature, subjectively appears grey, even though its objective physical spectrum peaks in the infrared range. When it becomes a little hotter, it appears dull red. As its temperature increases further it becomes yellow, white, and ultimately blue-white.

Figure 1.1 : Blackbody Radiation. When heated, all objects emit electromagnetic radiation whose wavelength (and color) depends on the temperature of the object. A relatively low-temperature object, such as a horseshoe forged by a blacksmith, appears red, whereas a higher-temperature object, such as the surface of the sun, appears yellow or white. Images used with permission from Wikipedia.
Blackbody radiation has a characteristic, continuous frequency spectrum that experimentally depends only on the body's temperature. In fact, we can be much more precise:
A body emits radiation at a given temperature and frequency exactly as well as it absorbs the same radiation.
This statement was proved by Gustav Kirchhoff: the essential point is that if we instead suppose a particular body can absorb better than it emits, then in a room full of objects all at the same temperature, it will absorb radiation from the other bodies better than it radiates energy back to them. This means it will get hotter, and the rest of the room will grow colder, contradicting the second law of thermodynamics. Thus, a body must emit radiation exactly as well as it absorbs the same radiation at a given temperature and frequency in order to not violate the second law of thermodynamics.
Any body at any temperature above absolute zero will radiate to some extent, the intensity and frequency distribution of the radiation depending on the detailed structure of the body. To begin analyzing heat radiation, we need to be specific about the body doing the radiating: the simplest possible case is an idealized body which is a perfect absorber, and therefore also (from the above argument) a perfect emitter. So how do we construct a perfect absorber in the laboratory? In 1859 Kirchhoff had a good idea: a small hole in the side of a large box is an excellent absorber, since any radiation that goes through the hole bounces around inside, a lot getting absorbed on each bounce, and has little chance of ever getting out again. So, we can do this in reverse: have an oven with a tiny hole in the side, and presumably the radiation coming out the hole is as good a representation of a perfect emitter as we’re going to find (Figure 1.2
).
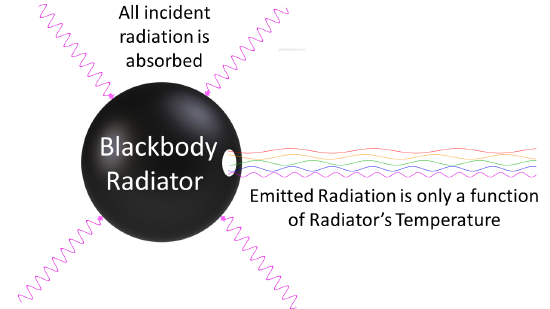
Figure 1.2 : Blackbody radiator is any object that is a perfect emitter and a perfect absorber of radiation.
By the 1890’s, experimental techniques had improved sufficiently that it was possible to make fairly precise measurements of the energy distribution of blackbody radiation. In 1895, at the University of Berlin, Wien and Lummer punched a small hole in the side of an otherwise completely closed oven, and began to measure the radiation coming out. The beam coming out of the hole was passed through a diffraction grating, which sent the different wavelengths/frequencies in different directions, all towards a screen. A detector was moved up and down along the screen to find how much radiant energy was being emitted in each frequency range. They found a radiation intensity/frequency curve close to the distributions in Figure 1.3.
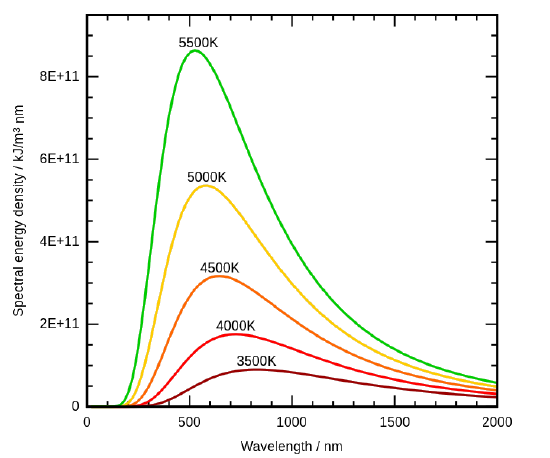
Figure 1.3 : Graphic representation of spectral distribution of blackbody radiation at different temperatures. (CC-SA-BY 3.0; 4C). The Stefan-Boltmann’s Law is observed as the increase in the emission amplitude with increasing temperature and the Wien’s Displacement Law is observed as the shift to smaller wavelength with increasing temperature.
By measuring the blackbody emission curves at different temperatures (Figure 1.3 ), they were also able to construct two important phenomenological Laws (i.e., formulated from experimental observations, not from basic principles of nature): Stefan-Boltmann’s Law and Wien’s Displacement Law.
Not all radiators are blackbody radiators
The radiation of a blackbody radiator is produced by the thermal activity of the material, not the nature of the material, nor how it got thermally excited. Some examples of blackbodies include incandescent light bulbs, stars, and hot stove tops. The emission appears as a continuous spectrum (Figure 1.3 ) with multiple coexisting colors. However, not every radiator is a blackbody radiator. For example, the emission of a fluorescence bulb is not one. The following spectrum show the distribution of light from a fluoresce light tube and is a mixture of discrete bands at different wavelengths of light in contrast to the continuous spectra in Figure 1.3
for blackbody radiators.
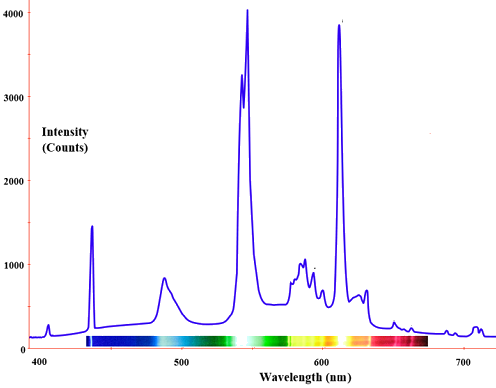
Fluorescent lighting spectrum with emission peaks. Graph of Intensity (counts) vs. Wavelength (nm) in the visible spectrum. (CC BY-SA 2.5; Deglr6328 and H Padleckas).
Fluorescent light bulbs contain a mixture of inert gases (usually argon and neon) together with a drop of mercury at low pressure. A different mix of visible colors blend to produce a light that appears to us white with different shadings.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|