


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-10-2019
Date: 29-10-2020
Date: 29-8-2019
|
If a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to a heteroatom (in organic chemistry, this term generally refers to oxygen, nitrogen, sulfur, or one of the halogens), the functional group is considered to be one of the ‘carboxylic acid derivatives’, a designation that describes a grouping of several functional groups. The eponymous member of this grouping is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl (OH) group.
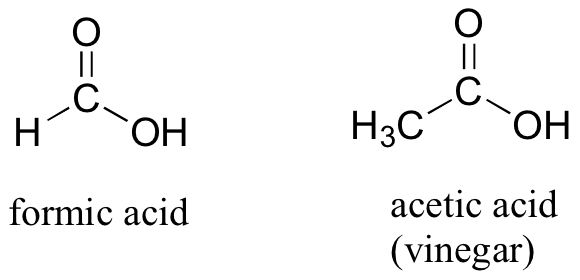
As the name implies, carboxylic acids are acidic, meaning that they are readily deprotonated to form the conjugate base form, called a carboxylate (much more about carboxylic acids in the acid-base chapter!).
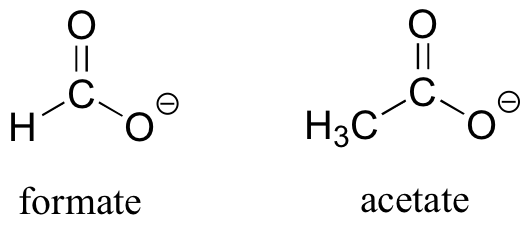
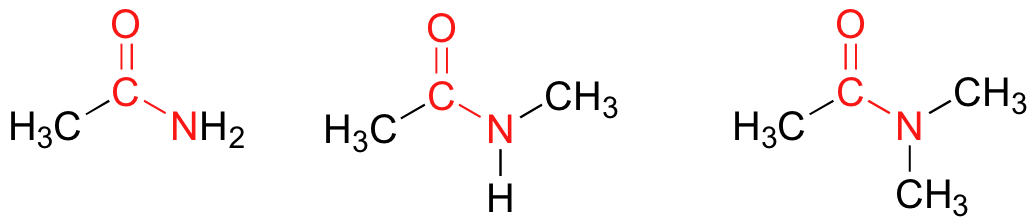
In amides, the carbonyl carbon is bonded to a nitrogen. The nitrogen in an amide can be bonded either to hydrogens, to carbons, or to both. Another way of thinking of an amide is that it is a carbonyl bonded to an amine.
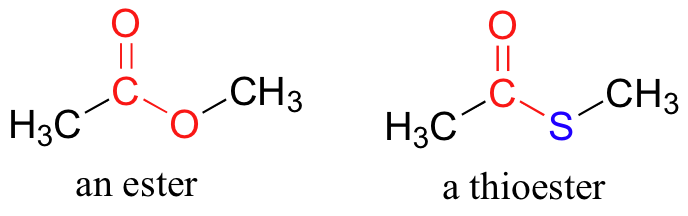
In esters, the carbonyl carbon is bonded to an oxygen which is itself bonded to another carbon. Another way of thinking of an ester is that it is a carbonyl bonded to an alcohol. Thioesters are similar to esters, except a sulfur is in place of the oxygen.
In an acyl phosphate, the carbonyl carbon is bonded to the oxygen of a phosphate, and in an acid chloride, the carbonyl carbon is bonded to a chlorine.

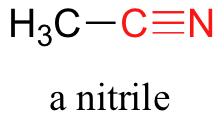
Finally, in a nitrile group, a carbon is triple-bonded to a nitrogen. Nitriles are also often referred to as cyano groups.
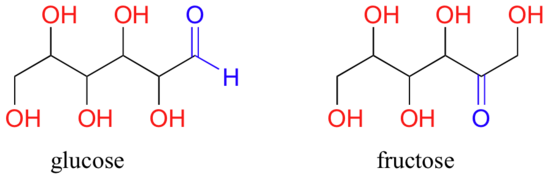
A single compound often contains several functional groups. The six-carbon sugar molecules glucose and fructose, for example, contain aldehyde and ketone groups, respectively, and both contain five alcohol groups (a compound with several alcohol groups is often referred to as a ‘polyol’).
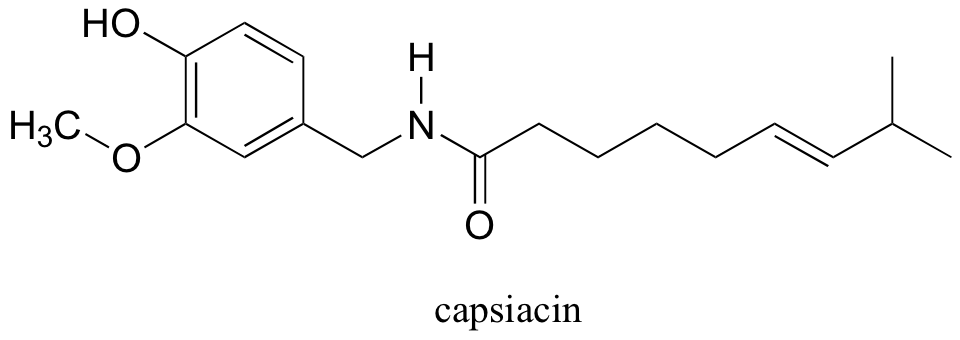
Capsaicin, the compound responsible for the heat in hot peppers, contains phenol, ether, amide, and alkene functional groups.
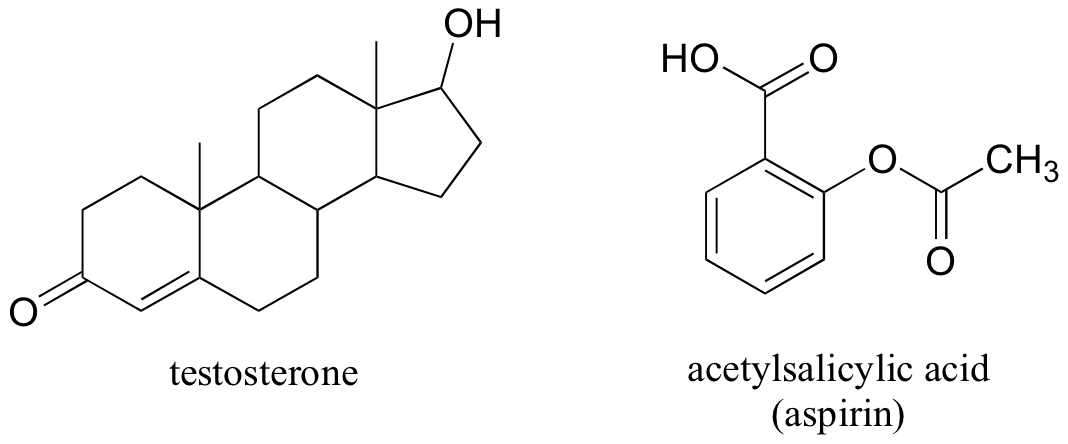
The male sex hormone testosterone contains ketone, alkene, and secondary alcohol groups, while acetylsalicylic acid (aspirin) contains aromatic, carboxylic acid, and ester groups.
While not in any way a complete list, this section has covered most of the important functional groups that we will encounter in biological and laboratory organic chemistry. The table on the inside back cover provides a summary of all of the groups listed in this section, plus a few more that will be introduced later in the text.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|