


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-9-2017
Date: 9-5-2016
Date: 27-7-2017
|
OXIDATION OF n-BUTANE (Acetic Acid and Acetaldehyde)
The oxidation of n-butane represents a good example illustrating the effect of a catalyst on the selectivity for a certain product. The noncatalytic oxidation of n-butane is nonselective and produces a mixture of oxygenated compounds including formaldehyde, acetic acid, acetone, and alcohols. Typical weight % yields when n-butane is oxidized in the vapor phase at a temperature range of 360–450°C and approximately 7 atmospheres are: formaldehyde 33%, acetaldehyde 31%, methanol 20%, acetone 4%, and mixed solvents 12%.
On the other hand, the catalytic oxidation of a n-butane, using either cobalt or manganese acetate, produces acetic acid at 75–80% yield. Byproducts of commercial value are obtained in variable amounts. In the Celanese process, the oxidation reaction is performed at a temperature range of 150–225°C and a pressure of approximately 55 atmospheres.
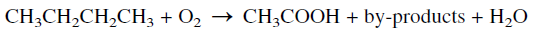
The main by-products are formic acid, ethanol, methanol, acetaldehyde, acetone, and methylethyl ketone (MEK). When manganese acetate is used as a catalyst, more formic acid (≈25%) is obtained at the expense of acetic acid.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|