


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-2-2018
Date: 25-6-2020
Date: 13-3-2017
|
Methane hydrates
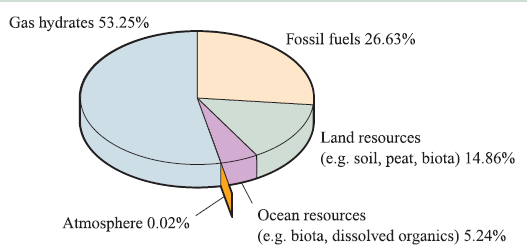
A gas hydrate is an example of a clathrate, a crystalline solid comprising a host (a three-dimensional assembly of H2O molecules which form cage-like arrays) and guest molecules (small molecules such as CH4 which occupy the cavities in the host lattice). Gas hydrates occur naturally in the Arctic and in deep-sea continental margins, and their importance lies in their ability to trap gases within crystalline masses, thereby acting rather like natural gas ‘storage tanks’. It is possible that such deposits could be tapped for fuel sources, but on the other hand, any uncontrolled release of the huge amounts of CH4 that is presently trapped inside these clathrates could add to the ‘greenhouse’ effect. The total amount of naturally occurring organic compound-based carbon on Earth is estimated to be about 19 000 ≈ 1015 t. In addition to this, carbon occurs widely in inorganic minerals such as carbonates. The chart opposite shows the relative importance of methane hydrates as a potential source of carbon from organic-based carbon materials.




|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|