

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Halides of selenium and tellurium
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 451
13-3-2017
4651
Halides of selenium and tellurium
In contrast to sulfur chemistry where dihalides are well established, the isolation of dihalides of selenium and tellurium has only been achieved for SeCl2 and SeBr2 (reactions 1.1 and 1.2). Selenium dichloride is a thermally unstable red oil; SeBr2 is a red-brown solid.
 (1.1)
(1.1)
 (1.2)
(1.2)
Table 1.1 lists selected properties of SeF4, SeF6, TeF4 and TeF6. Selenium tetrafluoride is a good fluorinating agent; it is a liquid at 298K and (compared with SF4) is relatively convenient to handle. It is prepared by reacting SeO2 with SF4.
Table 1.1 Selected properties of the fluorides of selenium and tellurium.

Combination of F2 and Se yields SeF6 which is thermally stable and relatively inert. The tellurium fluorides are similarly prepared, TeF4 from TeO2 and SF4 (or SeF4), and TeF6 from the elements. In the liquid and gas phases, SeF4 contains discrete molecules (Figure 1.1a) but in the solid state, significant intermolecular interactions are present. However, these are considerably weaker than in TeF4, in which the formation of Te_F_Te bridges leads to a polymeric structure in the crystal (Figure 1.1b).

Fig. 1.1 (a) The structure of SeF4 in the gas and liquid phases; (b) in the solid state, TeF4 consists of polymeric chains; (c) the structure of the molecular Se4Cl16-unit present in the crystal lattice of SeCl4. Colour code: Se, yellow; Te, blue; F and Cl, green.
Fluorine-19 NMR spectroscopic studies of liquid SeF4 have shown that the molecules are stereochemically nonrigid. The structures of SeF6 and TeF6 are regular octahedra. Tellurium hexafluoride is hydrolysed by water to telluric acid, H6TeO6, and undergoes a number of exchange reactions such as reaction 1.3. It is also a fluoride acceptor, reacting with alkali metal fluorides and [Me4N]F under anhydrous conditions (equation 1.4).
 (1.3)
(1.3)
 (1.4)
(1.4)
The[TeF7]- ion has a pentagonal bipyramidal structure (1.1) although in the solid state, the equatorial F atoms deviate slightly from the mean equatorial plane. In [TeF8]2- , 1.2, vibrational spectroscopic data are consistent with the Te centre being in a square-antiprismatic environment.

(1.1) (1.2)
In contrast to S, Se and Te form stable tetrachlorides, made by direct combination of the elements. Both the tetrachlorides are solids (SeCl4, colourless, subl. 469 K; TeCl4 yellow, mp 497 K, bp 653 K) which contain tetrameric units, depicted in Figure 1.1c for SeCl4. The E_Cl (E = Se or Te) bonds within the cubane core are significantly longer than the terminal E_Cl bonds; e.g. Te_Cl = 293 (core) and 231 (terminal) pm. Thus, the structure may also be described in terms of [ECl3] and Cl- ions. A cubane contains a central cubic (or near-cubic) arrangement of atoms. The [SeCl3] and [TeCl3] cations are also formed in reactions with Cl- acceptors, e.g. reaction 1.5.
 (1.5)
(1.5)
Both SeCl4 and TeCl4 are readily hydrolysed by water, but with group 1 metal chlorides in the presence of concentrated HCl, yellow complexes such as K2[SeCl6] and K2[TeCl6] are formed.
Reaction 1.6 is an alternative route to [TeCl6]2-, while [SeCl6]2- is formed when SeCl4 is dissolved in molten SbCl3 (equation 1.7).
 (1.6)
(1.6)
 (1.7)
(1.7)
The [SeCl6]2- and [TeCl6]2- ions usually (see below) possess regular octahedral structures (Oh symmetry), rather than the distorted structure (with a stereochemically active lone pair) that would be expected on the basis of VSEPR theory. In contrast, [SeF6]2-has a distorted octahedral structure. On going from [SeF6]2-to [SeCl6]2-, the change from a distorted to regular octahedral structure can be attributed to a decrease in the stereochemical activity of the lone pair as the steric crowding of the ligands increases. The same trend is seen on going from [BrF6]- (regular octahedral) to [IF6]- (distorted octahedral) as the size of the central atom increases and relieves steric congestion. A word of caution, however: in the solid state, the counter-ion can influence the structure of the anion. For example, in [H3N(CH2)3NH3][TeCl6], the [TeCl6]2- has approximately C2v symmetry, and in [tBuNH3]2[TeBr6], the [TeBr6]2- ion has approximately C3v symmetry. For the octahedral anions, a molecular orbital scheme can be developed (Figure 1.1) that uses only the valence shell 4p (Se) or 5p (Te) orbitals. Combined with six Cl 3p orbitals, this leads to seven occupied MOs in [ECl6]2- (E=Se, Te), of which four have bonding character, two have non-bonding character, and one has antibonding character. The net number of bonding MOs is therefore three, and the net E_Cl bond order is 0.5.
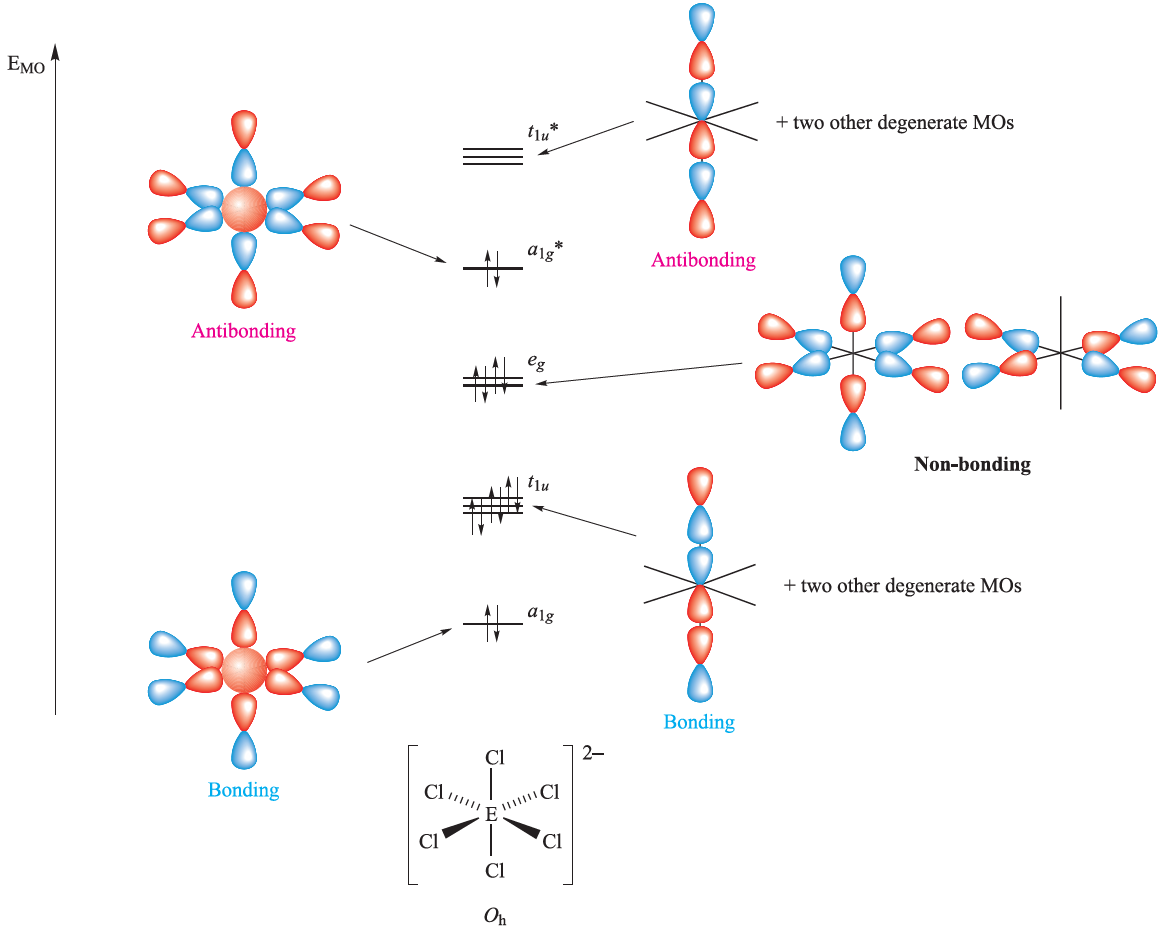
Fig. 1.1 An MO diagram for octahedral [ECl6]2- (E=Se or Te) using a valence set of 4s and 4p orbitals for Se or 5s and 5p orbitals for Te.
Tellurium forms a series of subhalides, e.g. Te3Cl2 and Te2Cl, the structures of which can be related to the helical chains in elemental Te. When Te is oxidized to Te3Cl2, oxidation of one in three Te atoms occurs to give polymer 1.3.

(1.3)
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















