

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Chloramine and hydroxylamine
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 398
19-2-2018
2376
Chloramine and hydroxylamine
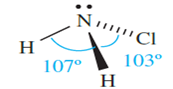
The reactions of NH3 and Cl2 (diluted with N2) or aqueous NaOCl yield chloramine the compound responsible for the odour of water containing nitrogenous matter that has been sterilized with Cl2. Chloramine is unstable, and violently explosive, and is usually handled in dilute solutions (e.g. in H2O or Et2O). Its reaction with Me2NH yields the rocket fuel 1,1-dimethylhydrazine.
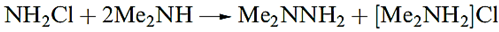

Reaction below is one of several routes to hydroxylamine, NH2OH, which is usually handled as a salt (e.g. the sulfate) or in aqueous solution. The free base can be obtained from its salts by treatment with NaOMe in MeOH.

Pure NH2OH forms white, hygroscopic crystals which melt at 306K and explode at higher temperatures. It is a weaker base than NH3 or N2H4. Many of its reactions arise from the great variety of redox reactions in which it takes part in aqueous solution, e.g. it reduces Fe(III) in acidic solution but oxidizes Fe(II) in the presence of alkali .
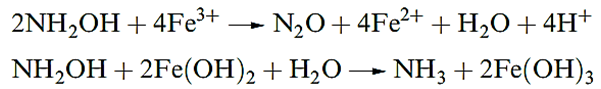
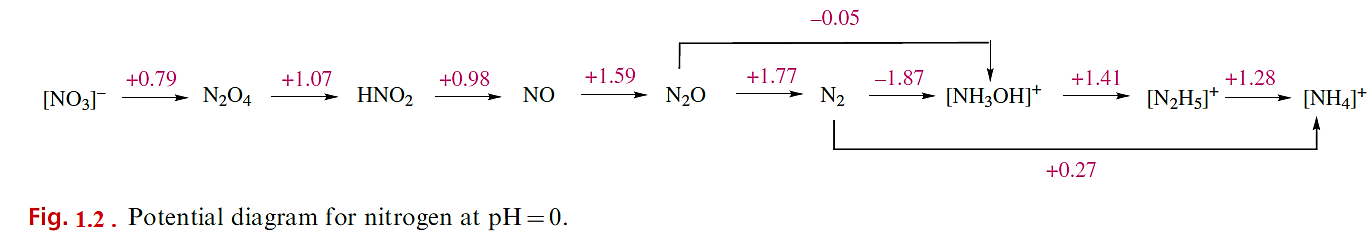
More powerful oxidizing agents (e.g. [BrO3]-) oxidize NH2OH to HNO3. The formation of N2O in most oxidations of NH2OH is an interesting example of the triumph of kinetic over thermodynamic factors. Consideration of the potential diagram in Figure 1.2 shows that, on thermodynamic grounds, the expected product from the action of weak oxidizing agents on
[NH3OH] (i.e. NH2OH in acidic solution) would be N2, but it seems that the reaction occurs by steps below. A use of NH2OH is as an antioxidant in photographic developers.

Figure 1.2 also shows that, at pH= 0, [NH3OH] is unstable with respect to disproportionation into N2 and [NH4] or [N2H5]; in fact, hydroxylamine does slowly decompose to N2 and NH3.
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















