


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 13-7-2016
Date: 4-9-2016
Date: 11-8-2016
|
Heat Capacity Ratio
To find CP/CV of a gas, one sometimes uses the following method. A certain amount of gas with initial temperature T0, pressure P0, and volume V0, is heated by a current flowing through a platinum wire for a time t. The experiment is done twice: first at a constant volume V0 with the pressure changing from P0 to P1, and then at a constant pressure with the volume changing from V0 to V1 The time t is the same in both experiments. Find the ratio CP/CV (the gas may be considered ideal).
SOLUTION
If the gas is heated at constant volume, then the amount of heat Q transferred to the gas is
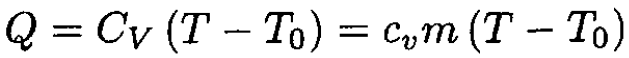 (1)
(1)
where cv is the heat capacity by weight of the gas, m is the mass, and T is the temperature at pressure P1. Using the ideal gas law at the beginning and end of heating gives
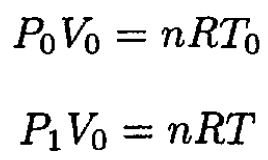 (2)
(2)
where n is the number of moles of the gas. From (1) and (2),
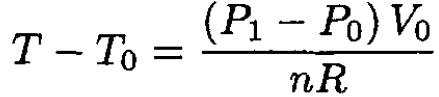 (3)
(3)
and
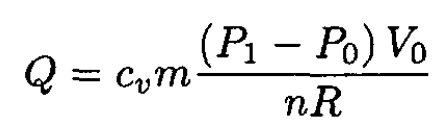 (4)
(4)
For heating at constant pressure,
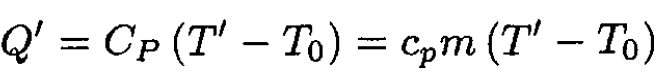 (5)
(5)
Similarly,
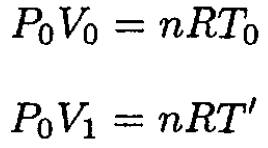 (6)
(6)
So
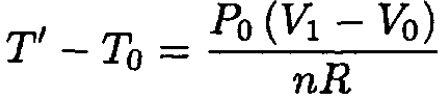 (7)
(7)
and
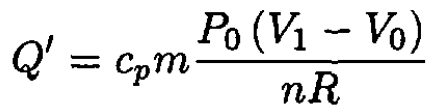 (8)
(8)
Since the time t during which the current flows through the wire is the same in both experiments, the amount of heat transferred to the gas is also the same: Q' = Q. Equating (4) and (8), we obtain
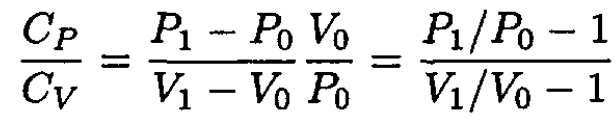 (9)
(9)



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
قسم الشؤون الفكرية والثقافية يجري اختبارات مسابقة حفظ دعاء أهل الثغور
|
|
|