


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-3-2016
Date: 10-3-2016
Date: 18-3-2016
|
Clostridium perfringens
1- Introduction
Clostridium perfringens is a pathogenic bacteria, which causes foodborne diseases classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) into two risk groups. The very common disease caused by the strains of type A is classified in risk group III: “diseases of moderate hazard usually not life threatening, normally of short dura-tion without substantial sequelae, causing symptoms that are self-limiting but can cause severe discomfort”.
The much more rare disease caused by the strains of type C (necrotic enteritis) is classified in risk group IB: “diseases of severe hazard for restricted population; life threatening or resulting in substantial chronic sequelae or presenting effects of long duration”.
1.1 Main characteristics of C. perfringens
According to Rainey et al. (2009) the cells of C. perfringens are Gram-positive rods, nonmotile, strictly anaerobic, catalase-negative. Growth is stimulated by the presence of a fermentable carbohydrate and is not inhibited by 20% bile. About 75% of the strains form capsules, predominantly composed of polysaccharides. They are spore-forming but spores are rarely seen in vivo or in vitro. When present they are oval, central or subterminal and distend the sporangia. According to ICMSF (1996) they rarely sporulate in culture but spores are produced readily in the intestine. According to Bates (1997) the optimal pH for growth of C. perfringens is 6.0–7.0 and the minimum is 5.5.
The characteristics most used for C. perfringens identification in food analysis is the sulfite reduction (positive), motility (negative), lactose fermentation (positive), gelatin hydrolysis (positive), and nitrate reduction (positive) (Labbe, 2001, Rhodehamel and Harmon, 2001). However, Rainey et al. (2009) reports 11 to 39% of strain negative for nitrate reduction.
One of the most striking characteristics of C. perfringens is the ability to grow actively at high temperatures: maximum of 50°C, optimal between 43 and 47°C, generation time of only 10 min at 45°C (Bates, 1997).
C. perfringens also ferments milk rapidly at 45°C, producing a typical fermentation within 18 h (stormy fermentation), due to the production of an acid curd and subsequent disruption of the curd by vigorous gas formation (Labbe, 2001, Rhodehamel and Harmon, 2001).
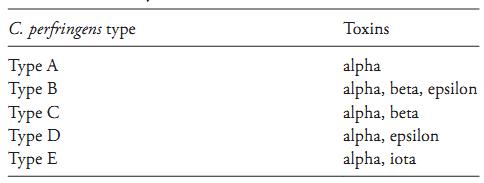
At low temperatures, on the contrary, vegetative cells of C. perfringens are very sensitive. ICMSF (1996) reports a minimum growth temperature of 12ºC and survival of only 6% of cells after 14 days in meat stored at minus 23°C. They die rapidly between zero and 10°C and storage for only a few days under refrigeration or at freezing temperatures may lead to a reduction of three to five logarithmic cycles in plate counts. They are also relatively sensitive to NaCl, grow well at concentrations of up to 2% but not at 6.5% NaCl. The minimum water activity is 0.93. C. perfringens is capable of producing a series of toxins and, based on their capacity to produce the four most lethal toxins (alpha, beta, epsilon and iota), the strains are classified into five types: A, B, C, D and E (Rainey et al., 2009), as shown in Table 1.
The alpha toxin is a phospholipase C that hydrolyzes lecithin (lecithinase), and is not associated with food-borne diseases. It causes gas gangrene (myonecrosis), an infection of muscle-tissue in animals and humans (Rainey et al., 2009). According to ICMSF (1996) the production of the alpha toxin can be observed in media that contain egg yolk, on which the colonies are surrounded by a turbid precipitation halo, typical of lecithinase activity. Inhibition of the activity of the
Table 1 Classification of C. perfringens into types based on the production of the alpha, beta, epsilon, and iota toxins (Rainey et al., 2009).
alpha toxin by its antitoxin, on the same culture media ( Nagler’s reaction), is a diagnostic test used for the confirmation of C. perfringens.
1.2 Epidemiology
Only types A and C of C. perfringens have been associated with diseases transmitted by foods, type A causing a common and relatively mild sickness and type C a rare but more severe illness (Bates, 1997).
1.2.1 C. perfringens type A food poisoning
The disease is the result of the action of an enterotoxin (Clostridium perfringens enterotoxin - CPE) produced in the intestine of the host after the ingestion of a high number of cells. The production of CPE is associated with the in vivo spore formation (Bates, 1997) and causes an intestinal disorder characterized by abdominal cramps and diarrhea. The incubation period is from eight to 22 hours, with the symptoms lasting for about 24 hours (in the elderly or sick people they may last for up to two weeks) (FDA/CFSAN, 2009).
In outbreaks, the diagnosis is confirmed by the detection of C. perfringens in suspected foods (counts ≥105/g) or in the feces of the patients (counts ≥106/g). The detection of CPE in feces of patients also confirms the diagnosis. It is important to highlight, however, that a high number of cells may not be detected in foods, since C. perfringens loses viability if the product is stored under refrigeration or frozen for prolonged periods of time. In these cases, the preparation of smears of the food sample followed by Gram-staining may help to confirm the presence of C. perfringens, the presence of which is evidenced by high numbers of typically large rods.
C. perfringens is widely distributed in nature, soil, dust and vegetation. It is also part of the normal flora of the intestinal tract of man and animals. Counts of about 103–104/g (dry weight) in the feces of healthy human adults are normal. When there is clinical involvement, counts are much higher, however, it is important to stress that in healthy elderly persons, it is common to find high number of spores.
The presence of small numbers of C. perfringens is not uncommon in raw meats, poultry, dehydrated soups and sauces, raw vegetables, and spices. Spores that sur-vive cooking may germinate and grow rapidly in foods that are inadequately refrigerated after cooking (Rhode-hamel and Harmon, 2001). Meats, meat products, and gravy are the foods most frequently implicated (FDA/CFSAN, 2009).
1.2.2 C. perfringens type C necrotic enteritis
The ingestion of foods contaminated with high numbers of C. perfringens type C cells causes necrotic enteritis, a very serious disease caused by the beta toxin, which results in necrosis of the intestine, sepsis or septicemia and, often, death. According to Bates (1997), the first cases occurred during the Second World War, due to the consumption of canned meat. In 1966–1967 new cases were reported in New Guinea, due to the consumption of contaminated pork meat. The disease was called the “pigbel” syndrome. The beta toxin is sensitive to trypsin, but, in New Guinea, the population is more vulnerable as a result of the high consumption of sweet potatoes. Sweet potatoes contain an inhibitor of trypsin which predisposes patients to the dis-ease, particularly children and adolescents. Hunger and malnutrition also reduce the level of trypsin in the gastrointestinal tract, thereby increasing susceptibility to the beta toxin. This has probably been one of the factors involved in the necrotic enteritis cases occurred during the Second World War. Sporadic cases have also been reported in Uganda, Malaysia, and Indonesia (Murrel, 1983).
1.3 Methods of analysis
There are several culture media available for the enumeration of C. perfringens in foods, such as Neomycin Blood Agar, Cycloserine Blood Agar, Sulfite Polymyxin Sulfadiazine Agar (SPS), Tryptone Sulfite Neomycin Agar (TSN), Shahidi Ferguson Perfringens Agar (SFP), Trypticase Soy Sheep Blood Agar (TSB), Oleandomycin Polymyxin Sulfadiazine Perfringens Agar (OPSP) and Tryptose Sulfite Cycloserine Agar (TSC), which may be used added with or without egg yolk (Labbe, 2001).
The selectivity of these media results from the incorporation of one or more antibiotics, added to inhibit several anaerobes and facultative anaerobes and, except in the case of the media containing blood, the differential characteristic common to all the other media is the presence of iron and sulfite. C. perfringens reduces sulfite to sulfide which reacts with iron and precipitates in the form of iron sulfide, producing black colonies. Among these media, SPS and TSN are considered excessively selective and little satisfactory, since they may inhibit several strains of C. perfringens. Some strains may grow on SPS, but without producing characteristically black colonies. On the other hand, SFP, OPSP and Neomycin Blood Agar are considered not selective enough and, for that reason, more adequate for situations in which C. perfringens constitutes the predominant microbiota in the food to be analyzed. Cycloserine Blood Agar seems adequate for the enumeration of C. perfringens, however, the data available that would allow better evaluation of its performance are still limited, since it hasn’t been routinely used in the microbiological examination of foods (Labbe, 2001).
TSC Agar is the medium most frequently used for the enumeration of C. perfringens by direct plating, constituting at the same time an excellent alternative for the enumeration of sulfite-reducing clostridia in general, due to its ability to suppress the growth of practically all facultative anaerobes that accompany clostridia in foods. It is important to emphasize, however, that TSC Agar (as well as the media used in the subsequent stages of the analysis) allows the growth and toxin production of C. botulinum, and for that reason, utmost care should be taken when performing the tests, to ensure the safety of both the analysts and the laboratory.
When used for enumerating C. perfringens, the TSC medium may be supplemented with egg yolk to verify the production of the alpha toxin (lecithinase). The incorporation of egg yolk, however, does not represent much of an advantage, since some facultative anaerobes may produce a similar reaction. Furthermore, not all C. perfringens strains produce a halo indicative of the lecithinase reaction, thus making it impossible to disregard and eliminate the colonies that are not surrounded by a halo. The halo produced by a colony may also be obscured by the halos of other colonies, making visualization of the reaction difficult.
The colonies presumptive for C. perfringens on TSC Agar should be confirmed by biochemical tests, with the following characteristics being considered the most recommended for this purpose: motility, lactose fermentation, hydrolysis of gelatin and nitrate reduction to nitrite.
The characteristic stormy fermentation at 46°C is recommended by BAM/FDA (Rhodehamel & Harmon, 2001) as a presumptive test for C. perfringens. Cultures that fail to exhibit “stormy fermentation” within 5 h are unlikely to be C. perfringens. An occasional strain may require 6 h or more, but this is a questionable result that should be confirmed by further testing.
References
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
ICMSF (International Commission on Microbiological Specifications for Foods) (1996) Microorganisms in Foods 5. Microbiological Specifications of Food Pathogens. London, Blackie Academic & Professional.
ICMSF (International Commission on Microbiological Specifications for Foods) (2002) Microorganisms in Foods 7. Microbiological Testing in Food Safety Management. New York, Kluwer Academic/Plenum Publishers.
Bates, J.R. (1997) Clostridium perfringens. In: Hocking, A.D. Arnold, G., Jenson, I., Newton, K. and Sutherland, P. (eds.). Foodborne Microorganisms of Public Health Significance. 5th edition. Chapter 13. Sydney, Trenear Printing Service Pty Limited. pp. 407–428.
Rainey, F.A., Hollen, B.J. & Small, A. (2009) Genus I Clostridium Prazmowski. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds.). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 738–828.
Rhodehamel, E.J. & Harmon, S.M. (2001) Clostridium perfringens. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 12. [Online] Silver Spring, Food and Drug Administration. Avail-able from: http://www.fda.gov/Food/ScienceResearch/Laborato-ryMethods/BacteriologicalAnalyticalManualBAM/default.htm [accessed 10th October 2011].
Labbe, R.G. (2001) Clostridium perfringens. In: Downes, F.P. & Ito, K. (eds.). Compendium of Methods for the Microbiological Examination of Foods. 4th edition. Washington, American Public Health Association. Chapter 34, pp. 325–330.
FDA/CFSAN (ed.) (2009) Foodborne Pathogenic Microorganisms and Natural Toxins Handbook “Bad Bug Book”. [Online] College Park, Food and Drug Administration, Center for Food Safety & Applied Nutrition. Available from: http://www.fda.gov/food/foodsafety/foodborneillness/foodborneillnessfoodbornepa-thogensnaturaltoxins/badbugbook/default.htm [accessed 10th October 2011].
Murrell, T.G.C. (1983). Pigbel in Papua New Guinea: An Ancient Disease Rediscovered. International Journal of Epidemiology, 12(2), 211–214.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|