


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-2-2016
Date: 12-2-2016
Date: 12-2-2016
|
HYDROFORMYLATION
The hydroformylation (oxo) reactions offer ways of converting a-olefins to aldehydes and/or alcohols containing an additional carbon atom.
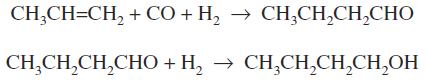
In the process (Fig. 1), the olefin in a liquid state is reacted at 27 to 30 MPa and 150 to 170oC in the presence of a soluble cobalt catalyst. The aldehyde and a lesser amount of the alcohol are formed and flashed off along with steam, and the catalyst is recycled. Conversions of over 97 percent are obtained, and the reaction is strongly exothermic. The carbon monoxide and hydrogen are usually in the form of synthesis gas.
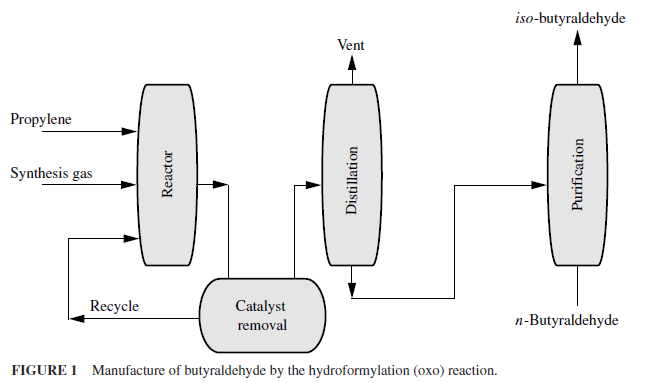
When propylene is used as the hydrocarbon, n- and iso-butyraldehyde are formed. This reaction is most frequently run with the C3 and C7 to C12 olefins. When C7 olefins are used, a series of dimethyl- and ethylhexanols and methyl heptanols are formed that are used as octyl alcohols to make
plasticizers and esters.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|