


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-4-2021
Date: 6-12-2015
Date: 2-4-2021
|
Recombination Pathways Adapted for Experimental Systems
KEY CONCEPTS
- Mitotic homologous recombination allows for targeted transformation.
- The Cre/lox and Flp/FRT systems allow for targeted recombination and gene knockout construction.
- The Flp/FRT system has been adapted to construct recyclable selectable markers for gene deletion.
Site-specific recombination not only has important biological roles, but has also been exploited to create targeted recombination events in experimental systems. Two classic examples of site-specific recombination have been adapted for experimental use: the Cre/lox and FLP/FRT systems.
The Cre/lox system is derived from bacteriophage P1. The Cre enzyme recognizes and cleaves lox sites. One of the most common uses of the Cre/lox system is in gene targeting in mice, as shown in FIGURE 1. Cre/lox can be used to conditionally turn off or turn on a gene in mice. A construct is designed that is flanked by lox sites, with the Cre gene under control of an inducible promoter that can be turned on by temperature, hormones, or in a tissue-specific pattern. Expression of Cre results in production of the Cre protein; the Cre protein then recognizes and cleaves the lox sites and promotes rejoining of the cut lox sites to leave behind a single lox site, with the material between the lox sites having been excised.
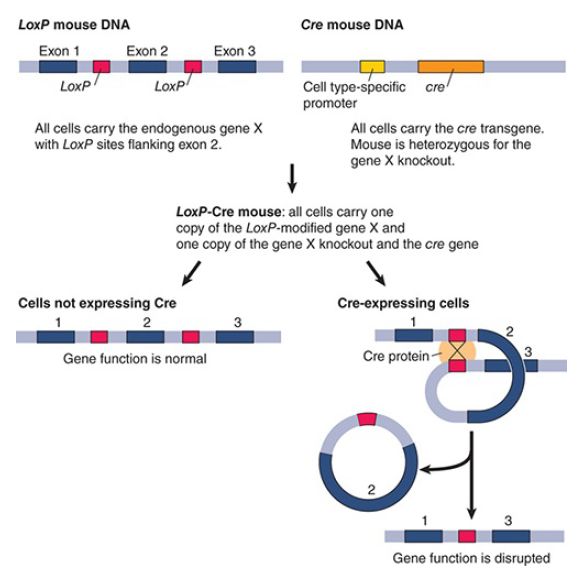
FIGURE 1. Using Cre/lox to make cell type–specific gene knockouts in mice. loxP sites are inserted into the chromosome to flank exon 2 of the gene X. The second copy of the X gene has been knocked out. The mouse formed with this construct is called the loxP mouse. Another mouse, called the Cre mouse, has the cre gene inserted into the genome. Adjacent to the cre gene is a promoter that directs expression of the cre gene only in certain cell types or in response to certain conditions. This mouse also carries a knockout of one copy of gene X. When the two mice are crossed, progeny that carry the loxP construct, the gene X knockout, and the cre gene are produced. When Cre protein is expressed in cells that activate the promoter, it catalyzes sitespecific recombination between the loxP sites, and exon 2 of gene X is deleted. This inactivates the one functional copy of gene X in those cells expressing Cre.
Data from H. Lodish, et al. Molecular Cell Biology, Fifth edition. W. H. Freeman & Company, 2003.
The Cre/lox system can be used to conditionally remove an exon from a mouse gene, resulting in a gene knockout , or it can fuse the gene of interest to a promoter and thereby control expression of the gene of interest. Expression of a gene in tissues where it is not normally expressed or at a time when the gene is not normally expressed is called ectopic expression. Ectopic expression studies can reveal information about gene redundancy, specificity, and cell autonomy.
Another system that has been adapted for experimental use is derived from the yeast S. cerevisiae. The 2-micron yeast plasmid is an autonomously replicating episome that is present in high copy numbers. The plasmid, which has no apparent benefit to the cell, is amplified through a site-specific recombination reaction that is carried out by a specialized recombinase known as Flp (flip). Flp recognizes inverted repeat sequences known as FRT (Flp recombinase target) sites. During replication, Flp-mediated recombination promotes rolling-circle replication that results in amplification of the 2-micron plasmid. The Flp/FRT system is used in Drosophila to induce site-specific mitotic recombination events that can be used to create homozygous mutations or to make conditional knockouts, as shown in FIGURE 2.
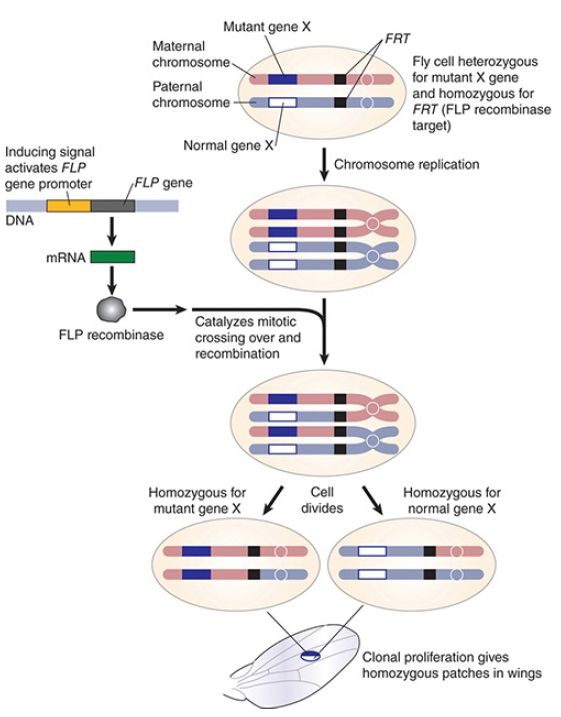
FIGURE 2 Using Flp/FRT to make homozygous recessive cells by homologous recombination. A fly is heterozygous for a mutant gene and homozygous insertion of the FRT site on the same chromosome. Induction of the Flp gene allows the FLP recombinase protein to be made. Flp recognizes the FRT site and makes a double-strand break, which promotes homologous recombination. Some of the recombination events occur by the double-strand break repair mechanism and result in crossing over. Following chromosome segregation, one daughter cell receives two mutant copies of the gene and the other daughter cell receives two normal copies of the gene. In the example shown, a patch of mutant cells is formed on the wing of a Drosophila. This technique allows assessment of a recessive mutant phenotype at a late stage in development.
Data from B. Alberts, et al. Molecular Biology of the Cell, Fourth edition. Garland Science, 2002.
To use the Flp/FRT system in Drosophila, FLP gene expression is regulated. When Flp is expressed, it cuts the FRT sites, which have been inserted on a chromosome where there is a gene of interest centromere-distal to the FRT site. The cutting of the FRT site, which is not 100% efficient, induces a DSB at the FRT site. The DSBs are repaired by homologous recombination, and some of them will result in crossing over. Depending on how the chromosomes then segregate, some cells will now be homozygous for the mutant gene. In genetic studies, the chromosome is often marked by a gene that affects a pigment, to give a visual readout for the recombination. The mitotic recombination uncovers the recessive pigmentation mutation and the mutant gene of interest, making them homozygous recessive. One use of this system is to see the effects of a lethal recessive mutation: When the zygote is homozygous recessive, the mutation will be lethal. If it is carried in the heterozygous state, though, the organism will be viable. Then the gene is rendered homozygous in clones of cells by induction of Flp, either by temperature or tissue-specific transcription regulation, enabling the investigator to ask about the effects of loss of the gene in specific cells at a specific time during development.
In recent years, Flp/FRT has been further adapted to construct recyclable selectable marker cassettes. In these systems, a selectable marker is placed between two flanking FRT sites. Also contained within the cassette is the FLP gene under the control of a regulatable promoter. Targeted integration of the FLP/FRT cassette is used to replace a locus of interest with the FLP marker cassette. Following integration, induced expression of the Flp recombinase catalyzes recombination between the flanking FRT sites, resulting in excision of the selectable marker cassette. This recyclable marker strategy is advantageous in diploid organisms because it allows for sequential rounds of targeted integration to make homozygous deletions of a gene of interest.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|