


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 7-6-2021
Date: 30-11-2015
Date: 25-12-2015
|
Annexins
Annexins are a family of structurally homologous, calcium- and membrane-binding proteins from eukaryotic sources including animals, plants, protists, and fungi. More than twenty distinct annexins have been characterized. Annexin homologues, notably 14-3-3 proteins, have also been identified through sequence similarities. Intracellularly, annexins vary in their organ, tissue, cell, and subcellular distribution and localization, and some annexins also occur extracellularly. The mechanism of release of annexins into the extracellular milieu is unclear because annexins lack signal sequences. Depending on the source, some annexins constitute a significant proportion (>1%) of total cell protein. Though their in vivo functions are not yet established, annexins have been implicated in many processes, including cell proliferation and differentiation, signal transduction, membrane trafficking and secretion, vesicle aggregation, cell adhesion, interactions with cytoskeletal elements, apoptosis, anticoagulation, phospholipase A2 inhibition, and ion channel activity or regulation. Phosphorylation may regulate some annexin-mediated processes. At least two annexins are major substrates for the tyrosine kinases, epidermal growth factor receptor, and retrovirus-encoded protein tyrosine kinase pp60v-src. Phosphorylations through other tyrosine kinases or serine/threonine kinases, for example, protein kinase C and cAMP-dependent protein kinase A, also have been observed.
Before annexins were recognized as a family, the proteins were identified in many laboratories by their calcium-dependent binding to particulate fractions or hydrophobic chromatographic column matrices. Initially these proteins were given diverse names, which reflected either their source or putative function, including lipocortins, calpactins, endonexins, placental anticoagulant proteins ) PAPs), chromobindins, calcimedins, and calelectrins. Subsequent sequence analysis revealed that many of these proteins have a common identity. The general term annexin subsequently was adopted in reference to their common ability to annex to membranes. In common nomenclature, individual annexins are designated by Roman numerals for example, annexin VII.
1. Macromolecular Interactions
The binding of calcium ions to annexins is relatively weak, with dissociation constants Kd in the millimolar-to-micromolar range. Complexation with membranes or other proteins increases their calcium-binding affinities. In vitro, annexins bind lanthanides and divalent cations, such as strontium, barium, and zinc. Calcium-dependent binding to membranes is high-affinity, and Kd is in the nanomolar range, whereas phospholipid monomers are bound poorly. In many systems, the annexin-membrane association is reversible upon addition of calcium chelators. In other systems, annexins behave more like integral membrane proteins. Typically, acidic phospholipids, such as phosphatidylserine, are strongly preferred by annexins, whereas binding to pure phosphatidylcholine membranes has not been observed. Such phospholipid binding preferences may target annexins to specific locations. The strong response of annexin V to extracellular exposure of phosphatidylserine is widely used as the basis of flow cytometric assays to detect membrane phospholipid asymmetry in apoptosis and other cellular processes.
In addition to their membrane lipid-binding properties, some annexins interact with other proteins. Some annexins interact with members of the EF-hand family, which suggests structural complementarity between the two protein families.
Annexins II and XI each form heterocomplexes in which the heavy chain is an annexin and the light chain resembles S-100 protein. Extracellular annexins have been described as cell surface receptors for some viruses (1, 2) and matrix proteins, such as collagen (reviewed in Ref. 3) and tenascin C (4). Annexin II is identified as a co-receptor for plasminogen/tissue plasminogen activator (reviewed in Ref. 5). Calcium-dependent lectin activity has been identified in some annexins that bind to specific sialoglycoproteins and glycosoaminoglycans, such as heparin (6, 7). The biological importance of this interaction is not yet understood, but it may be related to cell surface function.
2. Primary Structures and Molecular Evolution
Annexin sequences are comprised of two regions, a variable N-terminal region and a conserved C-terminal core region. The core region consists of four or eight canonical repeats of approximately 70 amino acid residues. Sequence homology exists between repeats within an individual annexin and between annexins. The sequence data suggest that gene duplication and subsequent gene fusion produced a four-domain ancestral precursor. Further gene fusion of two four-domain annexins produced annexin VI, which is unique in its possession of eight repeats. The core region is associated with the calcium-dependent phospholipid-binding properties of annexins. Each repeat contains a highly conserved stretch of amino acid residues with a consensus calcium-binding sequence (Lys/Arg)-Gly-X-Gly-Thr-X38-(Asp/Glu).
The variable N-terminal regions of annexins exhibit little homology and confer the distinct functional properties of individual annexins. Here the intron-exon structures are not highly conserved, whereas they are in the core region. The N-terminal region in annexins may consist of only a few amino acid residues or hundreds. Annexins VII (synexin) and XI have the largest and most hydrophobic N-terminal regions. These may considerably modify the properties of these proteins that arise from the common annexin core. In many annexins, phosphorylation sites occur within the N-terminal regions, as do some protein–protein interaction sites.
3. Crystal Structures of Annexins
Annexin crystal structures reveal a highly conserved alpha-helical fold in the C-terminal core region
characteristic of this family. Each basic repeating unit is made up of five a-helices, four of which run antiparallel, in a four-helix bundle and the fifth helix is perpendicular. The four-domain annexin structure is nearly planar, and the domains are arranged as a symmetrical array. The eight-domain annexin VI resembles two four-domain annexin structures that are approximately perpendicular to each other and are linked by a long, a-helical segment (8, 9). Most annexin crystal structures lack the variable N-terminal domains, which are intentionally truncated or lost through proteolysis. The only exception to date, that of full-length annexin I, reveals an unexpected juxtaposition between helices in the N-terminus and core domains, a finding that may suggest a mechanism for membrane aggregation (10).
In annexin crystal structures, numerous bound calcium ions are observed in loops along one surface of the protein molecule. These loops have structural motifs that are distinct from those in EF-hand proteins or C2-domain proteins. There are several structural motifs for these calcium-binding sites. The site corresponding to the consensus calcium-binding sequence has the highest affinity for calcium and structurally resembles a type of site observed in phospholipase A2. Other sites bind lanthanides more strongly than calcium. Annexin V (Fig. 1) adopts two distinct molecular conformations, depending on whether a tryptophan side chain in the third domain extends outward from the protein surface or lies buried within the protein core. Spectroscopic and biochemical evidence indicates that the two states are interrelated and may describe a calcium-dependent conformational change that influences membrane binding. The crystal structures of annexin V complexes with calcium and phospholipid head group analogs indicate that calcium ions are involved directly in the attachment to membranes (11).
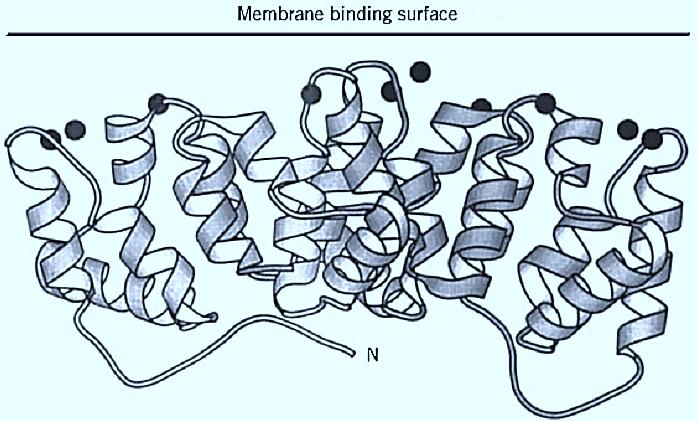
Figure 1. Ribbon diagram of annexin V (10). N-terminus as labeled, calcium ions as filled spheres.
4. Membrane-Bound Annexins
The membrane-bound structures of two annexins have been investigated by electron microscopy, and there is little evidence that the protein penetrates into the lipid bilayer. The molecular structures of membrane-bound annexins are essentially the same as in their crystals, except that the molecules reorient themselves so that their calcium-binding sites are in contact with the membrane (8, 12, 13). Several annexins exhibit calcium-dependent self-association and/or form two-dimensional arrays on membrane surfaces, a property that may underlie their biological functions (14, 15).
Structure-based mechanisms for annexin function have been proposed, but are not fully established. Two hypotheses have been presented to explain the annexin-induced calcium channel activity observed in vitro. In the “microscopic electroporation” model described for annexin V, the peripheral binding of the protein changes the electrostatic properties of the membrane (16). The calcium ion is translocated to the cytosol through a putative central pore in the annexin molecule. In an alternative model of calcium channel activity, based on hydra annexin XII, the protein hexamer inserts into the bilayer and creates a transmembrane structure resembling an inverted micelle (17 18, ). Experimental data have been offered to support both hypotheses, but the mechanism and physiological relevance of the channel activity remains controversial. Mechanistic models in which annexins restrict access to membrane phospholipids and/or surfaces have been proposed to explain their inhibition of phospholipase A2 (19) and thrombin (14), and various models have been suggested for processes, such as vesicle aggregation and membrane fusion. The in vivo functions of annexins remain under intensive investigation.
References
1. K. Hertogs et al. (1993) Virology 197, 549–557.
2. J. F. Wright, A. Kurosky, and S. Wasi (1994) Biochem. Biophys. Res. Commun. 198, 983–989.
3. K. von der Mark and J. Mollenhauer (1997) Cell. Mol. Life Sci. 53, 539–545.
4. C. Y. Chung and H. P. Erickson (1994) J. Cell. Biol. 126, 539–548.
5. K. A. Hajjar and J. S. Menell (1997) Ann. N.Y. Acad. Sci. 811, 337–349.
6. K. Kojima et al. (1996) J. Biol. Chem. 271, 7679–7685.
7. G. Kassam et al. (1997) J. Biol. Chem. 272, 15093–15100.
8. J. Benz et al. (1996) J. Mol. Biol. 260, 638–643.
9. H. Kawasaki, A. Avila-Sakar, C. E. Creutz, and R. H. Kretsinger (1996) Biochim. Biophys. Acta 1313, 277–282.
10. A. Rosengarth, V. Gerke, and H. Luecke (2001) J. Mol. Biol. 306, 489–498.
11. M. A. Swairjo et al. (1995) Nat. Struct. Biol. 2, 968–974.
12. A. Oloffson, V. Mallouh, and A. Brisson (1994) J. Struct. Biol. 113, 199–205.
13. D. Voges et al. (1994) J. Mol. Biol. 238, 199–213.
14. H. A. M. Andree et al. (1992) J. Biol. Chem. 267, 17907–17912.
15. C. Pigault et al. (1994) J. Mol. Biol. 236, 199–208.
16. P. Demange et al. (1994) Trends Biochem. Sci. 19, 272–276.
17. H. Luecke et al. (1995) Nature 378, 512–515.
18. S. E. Moss (1995) Nature 378, 446–447.
19. F. F. Davidson and E. A. Dennis (1989) Biochem. Pharmacol. 38, 3645–3651.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|