


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-9-2016
Date: 17-9-2020
Date: 8-1-2020
|
The Diels-Alder reaction is an example of an organic chemical reaction which does not proceed by either a polar or a free radical pathway, but rather a pericyclic reaction.
Although we do not expect you to be able to provide a detailed account of the mechanism of this reaction, you should learn enough about the Diels-Alder reaction to fulfil the objectives stated above. You will find it useful to contrast the mechanism of the Diels-Alder reaction with the polar and radical mechanisms studied earlier.
The unique character of conjugated dienes manifests itself dramatically in the Diels-Alder Cycloaddition Reaction. A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.
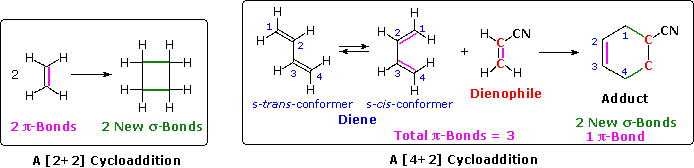
In the hypothetical ethylene dimerization on the left, each reactant molecule has a pi-bond (colored orange) occupied by two electrons. The cycloaddition converts these pi-bonds into new sigma-bonds (colored green), and this transformation is then designated a [2+2] cycloaddition, to enumerate the reactant pi-electrons that change their bonding location.
The Diels-Alder reaction is an important and widely used method for making six-membered rings, as shown on the right. The reactants used in such reactions are a conjugated diene, simply referred to as the diene, and a double or triple bond coreactant called the dienophile, because it combines with (has an affinity for) the diene. The Diels-Alder cycloaddition is classified as a [4+2] process because the diene has four pi-electrons that shift position in the reaction and the dienophile has two.
The Diels-Alder reaction is a single step process, so the diene component must adopt an s-cisconformation in order for the end carbon atoms (#1 & #4) to bond simultaneously to the dienophile. For many acyclic dienes the s-trans conformer is more stable than the s-cis conformer (due to steric crowding of the end groups), but the two are generally in rapid equilibrium, permitting the use of all but the most hindered dienes as reactants in Diels-Alder reactions. In its usual form, the diene component is electron rich, and the best dienophiles are electron poor due to electron withdrawing substituents such as CN, C=O & NO2. The initial bonding interaction reflects this electron imbalance, with the two new sigma-bonds being formed simultaneously, but not necessarily at equal rates.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|