


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-1-2020
Date: 17-1-2020
Date: 18-1-2020
|
The boiling points shown are for the "straight chain" isomers of which there is more than one. The first four alkanes are gases at room temperature, and solids do not begin to appear until about C17H36
, but this is imprecise because different isomers typically have different melting and boiling points. By the time you get 17 carbons into an alkane, there are unbelievable numbers of isomers!
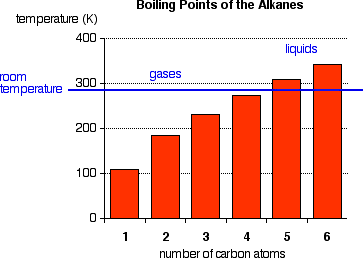
Cycloalkanes have boiling points that are approximately 20 K higher than the corresponding straight chain alkane.
There is not a significant electronegativity difference between carbon and hydrogen, thus, there is not any significant bond polarity. The molecules themselves also have very little polarity. A totally symmetrical molecule like methane is completely non-polar, meaning that the only attractions between one molecule and its neighbors will be Van der Waals dispersion forces. These forces will be very small for a molecule like methane but will increase as the molecules get bigger. Therefore, the boiling points of the alkanes increase with molecular size.
Where you have isomers, the more branched the chain, the lower the boiling point tends to be. Van der Waals dispersion forces are smaller for shorter molecules and only operate over very short distances between one molecule and its neighbors. It is more difficult for short, fat molecules (with lots of branching) to lie as close together as long, thin molecules.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|