


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-10-2020
Date: 5-7-2019
Date: 3-7-2019
|
Protons and other electrophiles are not the only reactive species that initiate addition reactions to carbon-carbon double bonds. Curiously, this first became evident as a result of conflicting reports concerning the regioselectivity of HBr additions. As noted earlier, the acid-induced addition of HBr to 1-butene gave predominantly 2-bromobutane, the Markovnikov Rule product. However, in some early experiments in which peroxide contaminated reactants were used, 1-bromobutane was the chief product. Further study showed that an alternative radical chain-reaction, initiated by peroxides, was responsible for the anti-Markovnikov product. This is shown by the following equations.
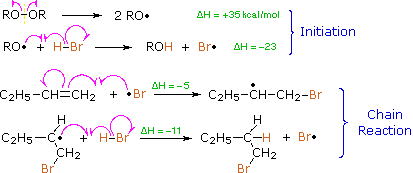
The weak O–O bond of a peroxide initiator is broken homolytically by thermal or hight energy. The resulting alkoxy radical then abstracts a hydrogen atom from HBr in a strongly exothermic reaction. Once a bromine atom is formed it adds to the π-bond of the alkene in the first step of a chain reaction. This addition is regioselective, giving the more stable carbon radical as an intermediate. The second step is carbon radical abstraction of another hydrogen from HBr, generating the anti-Markovnikov alkyl bromide and a new bromine atom. Each of the steps in this chain reaction is exothermic, so once started the process continues until radicals are lost to termination events.
This free radical chain addition competes very favorably with the slower ionic addition of HBr described earlier, especially in non-polar solvents. It is important to note, however, that HBr is unique in this respect. The radical addition process is unfavorable for HCl and HI because one of the chain steps becomes endothermic (the second for HCl & the first for HI).
Other radical addition reactions to alkenes have been observed, one example being the peroxide induced addition of carbon tetrachloride shown in the following equation
| RCH=CH2 + CCl4 (peroxide initiator) —> RCHClCH2CCl3 |
The best known and most important use of free radical addition to alkenes is probably polymerization. Since the addition of carbon radicals to double bonds is energetically favorable, concentrated solutions of alkenes are prone to radical-initiated polymerization, as illustrated for propene by the following equation. The blue colored R-group represents an initiating radical species or a growing polymer chain; the propene monomers are colored maroon. The addition always occurs so that the more stable radical intermediate is formed.
RCH2(CH3)CH· + CH3CH=CH2 —>RCH2(CH3)CH-CH2(CH3)CH· + CH3CH=CH2 —>RCH2(CH3)CHCH2(CH3)CH-CH2(CH3)CH· —> etc.
Anti-Markovnikov rule describes the regiochemistry where the substituent is bonded to a less substituted carbon, rather than the more substitued carbon. This process is quite unusual, as carboncations which are commonly formed during alkene, or alkyne reactions tend to favor the more substitued carbon. This is because substituted carbocation allow more hyperconjugation and indution to happen, making the carbocation more stable.
This process was first explained by Morris Selig Karasch in his paper: 'The Addition of Hydrogen Bromide to Allyl Bromide' in 1933.1 Examples of Anti-Markovnikov includes Hydroboration-Oxidation and Radical Addition of HBr. A free radical is any chemical substance with unpaired electron. The more substituents the carbon is connected to, the more substituted is that carbon. For example: Tertiary carbon (most substituted), Secondary carbon (medium substituted), primary carbon (least substituted)
Anti-Markovnikov Radical Addition of Haloalkane can ONLY happen to HBr and there MUST be presence of Hydrogen Peroxide (H2O2). Hydrogen Peroxide is essential for this process, as it is the chemical which starts off the chain reaction in the initiation step. HI and HCl cannot be used in radical reactions, because in their radical reaction one of the radical reaction steps: Initiation is Endothermic, as recalled from Chem 118A, this means the reaction is unfavorable. To demonstrate the anti-Markovnikov regiochemistry, I will use 2-Methylprop-1-ene as an example below:
.gif?revision=1&size=bestfit&width=316&height=115)
Hydrogen Peroxide is an unstable molecule, if we heat it, or shine it with sunlight, two free radicals of OH will be formed. These OH radicals will go on and attack HBr, which will take the Hydrogen and create a Bromine radical. Hydrogen radical do not form as they tend to be extremely unstable with only one electron, thus bromine radical which is more stable will be readily formed.
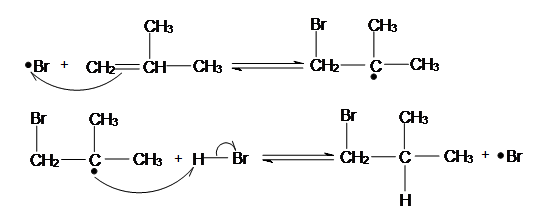
The Bromine Radical will go on and attack the LESS SUBSTITUTED carbon of the alkene. This is because after the bromine radical attacked the alkene a carbon radical will be formed. A carbon radical is more stable when it is at a more substituted carbon due to induction and hyperconjugation. Thus, the radical will be formed at the more substituted carbon, while the bromine is bonded to the less substituted carbon. After a carbon radical is formed, it will go on and attack the hydrogen of a HBr, which a bromine radical will be formed again.
There are also Termination Steps, but we do not concern about the termination steps as they are just the radicals combining to create waste products. For example two bromine radical combined to give bromine. This radical addition of bromine to alkene by radical addition reaction will go on until all the alkene turns into bromoalkane, and this process will take some time to finish.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
بمناسبة مرور 40 يومًا على رحيله الهيأة العليا لإحياء التراث تعقد ندوة ثقافية لاستذكار العلامة المحقق السيد محمد رضا الجلالي
|
|
|