


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-10-2018
Date: 4-11-2018
Date: 26-11-2018
|
Reactivity of group 13 elements
Boron is inert under normal conditions except for attack by F2. At high temperatures, it reacts with most non-metals (exceptions include H2), most metals and with NH3; the formations of metal borides and boron nitride are of particular importance.
The reactivities of the heavier group 13 elements contrast with that of the first member of the group. Aluminium readily oxidizes in air (see above); it dissolves in dilute mineral acids but is passivated by concentrated HNO3. Aluminium reacts with aqueous NaOH or KOH liberating H2.

Reactions of Al with halogens at room temperature or with N2 on heating give the Al(III) halides or nitride. Aluminium is often used to reduce metal oxides, e.g. in the thermite process which is highly exothermic.

Gallium, indium and thallium dissolve in most acids to give salts of Ga(III), In(III) or Tl(I), but only Ga liberates H2 from aqueous alkali. All three metals react with halogens at, or just above, 298 K; the products are of the type MX3 with the exceptions of reactions 12.6 and 12.7.
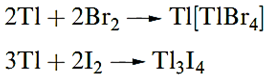



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|