


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-12-2018
Date: 1-4-2019
Date: 28-10-2018
|
Extraction Of the group 13
Of the group 13 elements, Al is of the greatest commercial importance with uses exceeding those of all metals except Fe; Figure 1.1 shows the dramatic rise in the production of Al in the US (the world’s largest producer) since 1930. Its isolation from the widely available aluminosilicate minerals is prohibitively difficult; bauxite and cryolite are the chief ores, and both are consumed in the extraction process. Crude bauxite is a mixture of oxides (impurities include Fe2O3, SiO2 and TiO2) and is purified using the Bayer process.
After addition of the crude ore to hot aqueous NaOH under pressure (which causes Fe2O3 to separate), the solution is seeded with Al2O3.3H2O and cooled, or is treated with a stream of CO2 to precipitate crystalline a-Al(OH)3. Anhydrous Al2O3 (alumina) is produced by the action of heat. Electrolysis of molten Al2O3 gives Al at the cathode, but the melting point (2345 K) is high, and it is more practical and economical to use a mixture of cryolite and alumina as the electrolyte with an operating temperature for the melt of 1220 K. The extraction is expensive in terms of the electrical power required, and Al production is often associated with hydroelectric schemes. The first steps in the extraction of boron from borax are its conversion to boric acid and then to the oxide.
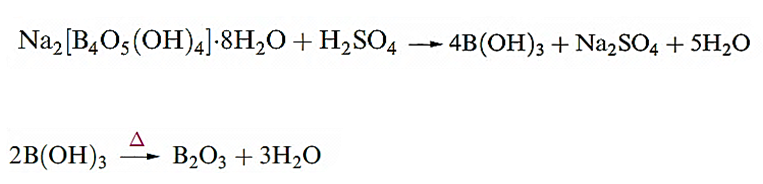
Boron of low purity is obtained by reduction of the oxide by Mg, followed by washing the product with alkali, hydrochloric acid and then hydrofluoric acid. The product is a very hard, black solid of low electrical conductivity which is inert towards most acids, but is slowly attacked by concentrated HNO3 or fused alkali. Pure boron is made by the vapour-phase reduction of BBr3 with H2, or by pyrolysis of B2H6 or BI3. At least four allotropes can be obtained under different conditions but transitions between them are extremely slow.

An increase in world production of Ga over the last part of the twentieth century (Figure 1.2) coincides with increased demand for gallium arsenide (GaAs) in components for electronic equipment. The main source of Ga is crude bauxite, in which Ga is associated with Al. Gallium is also obtained from residues from the Zn-processing industry. The development of the electronics industry has also led to a significant increase in the demand for indium. Indium occurs in the zinc sulfide ore sphalerite (also called zinc blende, see Figure 5.18) where, being a similar size to Zn, it substitutes for some of the Zn. The extraction of zinc from ZnS therefore provides indium as a by-product.
Recycling of In is becoming important, in particular where natural reserves of ZnS are low, e.g. in Japan. Thallium is obtained as a by-product of the smelting of Cu, Zn and Pb ores, although demand for the element is low (see below).

Fig. 1.2 World production and US consumption of gallium between 1975 and 2001. [Data: US Geological Survey.]



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|