


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-4-2019
Date: 10-3-2019
Date: 28-12-2018
|
Halides of Mg, Ca, Sr and Ba
The fluorides ofMg(II), Ca(II), Sr(II) andBa(II) are ionic, have high melting points, and are sparingly soluble in water, the solubility increasing slightly with increasing cation size (Ksp for MgF2, CaF2 , SrF2 and BaF2 = 7.42 × 10 -11, 1.46 × 10- 10, 4.33 × 10 -9 and 1.84 ×10 -7 respectively). Whereas MgF2 adopts a rutile lattice CaF2, SrF2 and BaF2 crystallize with the fluorite structure.
In contrast to the behaviour of BeF2, none of the later metal fluorides behaves as a Lewis acid. The structures of gaseous group 2 metal fluoride and later halide molecules are summarized in Table 1.1 and are the subject of ongoing theoretical interest. The term ‘quasilinear’ refers to a species for which the calculated energy difference between linear and bent structures (with a change in angle of >208) is less than 4 kJ mol-1. The most bent of the dihalides is BaF2.
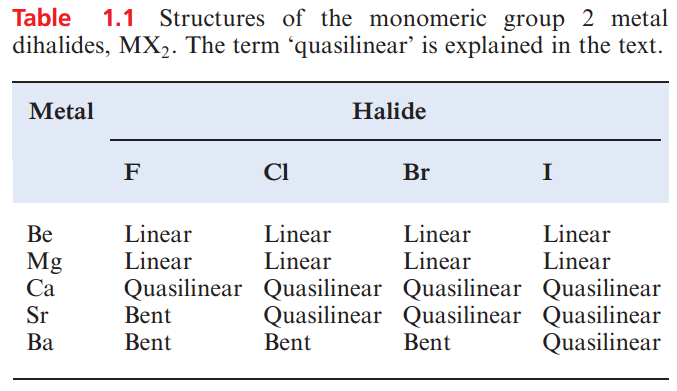
It has a bond angle in the region of 110–1268 (values come from a range of theoretical and experimental data) and the calculated energy to convert bent BaF2 to a linear molecule is ≈21 kJ mol-1. The preference for bent structures for the heaviest metals combined with F, Cl or Br (see Table 1.1) has been explained in terms of both ‘inverse (or core) polarization’ and the participation of d atomic orbitals for Ca, Sr and Ba. Inverse polarization occurs when the metal ion is polarizable and is polarized by F- or Cl-, or to a lesser extent, by Br-. This is represented in below diagram. The polarization is termed ‘inverse’ to distinguish it from the polarization of a large, polarizable anion by a cation.
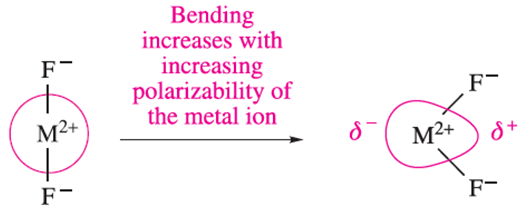
An alternative explanation focuses on the participation of d orbitals in the bonding in CaX2, SrX2 and BaX2. Table 1.1 shows that Be and Mg form only linear gaseous dihalides. These two metals have only s and p atomic orbitals available for bonding and the best M_X orbital overlap is achieved for a linear molecule. This is shown in below diagram for an np orbital on M with the out-of-phase combination of X…….X orbitals. For Ca, Sr and Ba, vacant 3d, 4d and 5d orbitals, respectively, are available, but can only overlap efficiently with orbitals on the X atoms if theMX2 molecule is bent. Two interactions must be considered as shown in below diagram (the axes are defined arbitrarily as shown). The out-of-phase combination of X…..X orbitals only overlaps efficiently with the dyz orbital of M if the MX2 molecule is bent; opening the molecule up to a linear shape ‘switches off’ this orbital interaction.
Although the interaction between the metal dz2 orbital and the in-phase combination of X…..X orbitals is most efficient when MX2 is linear, it is still effective when the molecule is bent. The inverse polarization and participation of d atomic orbitalsmay both contribute to the problem of bent MX2 molecules, and the explanation for the trend in shapes listed in Table 1.1 remains a matter for debate.†
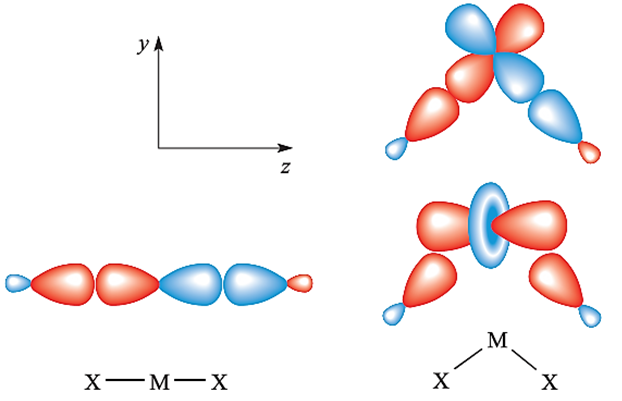
Magnesium chloride, bromide and iodide crystallize from aqueous solution as hydrates which undergo partial hydrolysis when heated. The anhydrous salts are, therefore, prepared by reaction in below.
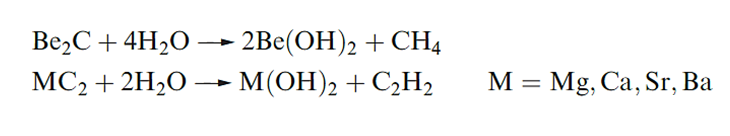
Anhydrous MCl2, MBr2 and MI2 (M= Ca, Sr and Ba) can be prepared by dehydration of the hydrated salts. These anhydrous halides are hygroscopic and CaCl2 is used as a laboratory drying agent and for road de-icing. In the solid state, many of the anhydrous halides possess complicated layer structures such as the CdI2 lattice. Most of these halides are somewhat soluble in polar solvents such as ethers or pyridine, and a number of crystalline complexes have been isolated.
Octahedral coordination has been confirmed by X-ray diffraction studies of complexes including trans-[MgBr2(py)4], trans-[MgBr2(THF)4], cis-[MgBr2(diglyme)(THF)] (Figure 1.1a) and trans-[CaI2(THF)4].
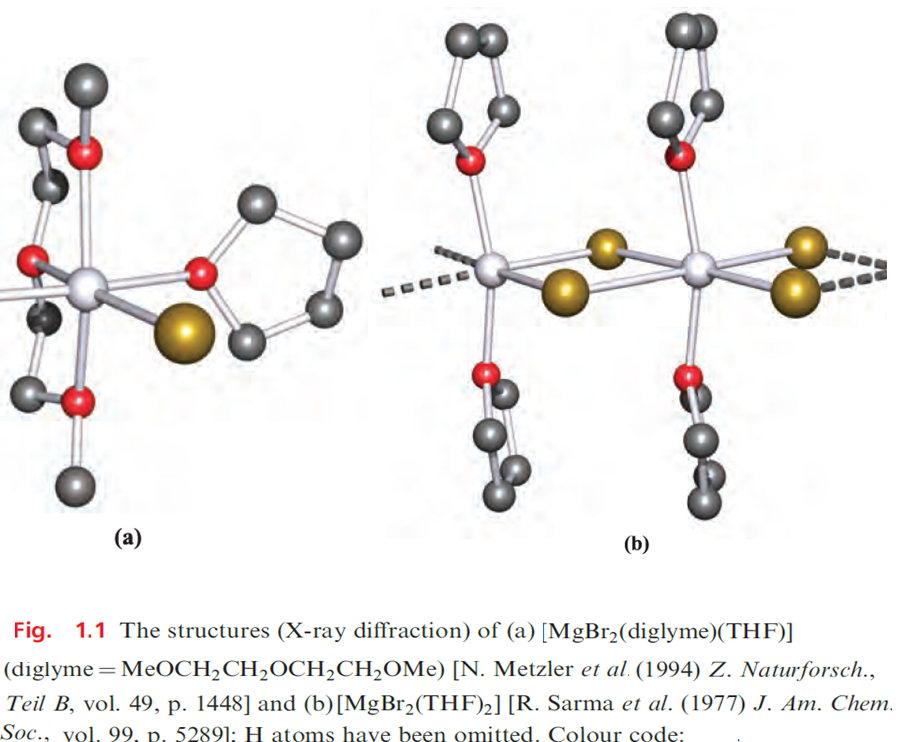
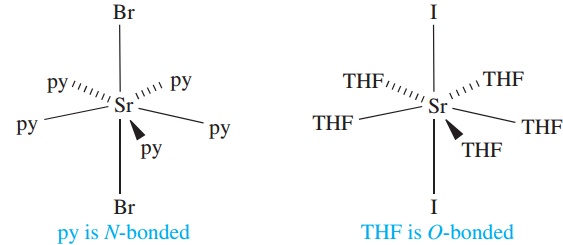
In [MgBr2(THF)2], octahedral coordination in the solid state is achieved by the formation of a chain structure (Figure 1.1b); py = pyridine, THF = tetrahydrofuran. The larger sizes of the heavier metals permit higher coordination numbers, e.g. pentagonal bipyramidal trans-[SrBr2(py)5], and trans-[SrI2(THF)5]. In organic chemistry, MgBr2 is used as a catalyst for esterification reactions, and MgBr2.2Et2O is commercially available, being a catalyst for the conversion of aliphatic epoxides to the corresponding ketones.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
واحات الحزام الأخضر تشهد توافد العوائل العراقية في أيام عيد الفطر المبارك
|
|
|