


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-9-2017
Date: 27-7-2017
Date: 18-9-2017
|
NAPHTHA-BASED CHEMICALS
Light naphtha containing hydrocarbons in the C5-C7 range is the preferred feedstock in Europe for producing acetic acid by oxidation.
Similar to the catalytic oxidation of n-butane, the oxidation of light naphtha is performed at approximately the same temperature and pressure ranges (170–200°C and ≈50 atmospheres) in the presence of manganese acetate catalyst. The yield of acetic acid is approximately 40 wt%.
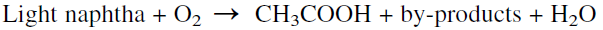
The product mixture contains essentially oxygenated compounds (acids, alcohols, esters, aldehydes, ketones, etc.). As many as 13 distillation columns are used to separate the complex mixture. The number of products could be reduced by recycling most of them to extinction.
Manganese naphthenate may be used as an oxidation catalyst. Rouchaud and Lutete have made an in-depth study of the liquid phase oxidation of n-hexane using manganese naphthenate. A yield of 83% of C1-C5 acids relative to n-hexane was reported. The highest yield of these acids was for acetic acid followed by formic acid. The lowest yield was observed for pentanoic acid.
In Europe naphtha is the preferred feedstock for the production of synthesis gas, which is used to synthesize methanol and ammonia . Another important role for naphtha is its use as a feedstock for steam cracking units for light olefins production. Heavy naphtha, on the other hand, is a major feedstock for catalytic reforming. The product reformate containing a high percentage of C6-C8 aromatic hydrocarbons is used to make gasoline. Reformates are also extracted to separate the aromatics as intermediates for petrochemicals.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|