

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Coordination Polymerization
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p 309
18-9-2017
4573
Coordination Polymerization
Polymerizations catalyzed with coordination compounds are becoming more important for obtaining polymers with special properties (linear and stereospecific). The first linear polyethylene polymer was prepared from a mixture of triethylaluminum and titanium tetrachloride (Ziegler catalyst) in the early 1950s. Later, Natta synthesized a stereoregular polypropylene with a Ziegler-type catalyst. These catalyst combinations are now called Zieglar-Natta catalysts.
In coordination polymerization, the bonds are appreciably covalent but with a certain percentage of ionic character. Bonding occurs between a transition metal central ion and the ligand (perhaps an olefin, a diolefin or carbon monoxide) to form a coordination complex. The complex reacts further with the ligand to be polymerized by an insertion mechanism.
Different theories about the formation of coordination complexes have been reviewed by Huheey. In recent years, much interest has been centered on using late transition metals such as iron and cobalt for polymerization.
Due to their lower electrophilicity, they have greater tolerence for polar functionality. It was found that the catalyst activity and the polymer branches could be modified by altering the bulk of the ligand that surrounds the central metal. Such a protection reduces chain-transfer reactions and results in a high molecular-weight polymer. An example of these catalysts are pyridine bis-imine ligands complexed with iron and cobalt salts.
Ziegler-Natta catalysts currently produce linear polyethylene (nonbranched), stereoregular polypropylene, cis-polybutadiene, and other stereoregular polymers.
In polymerizing these compounds, a reaction between α-TiCl3 and triethylaluminum produces a five coordinate titanium (III) complex arranged octahedrally. The catalyst surface has four Cl anions, an ethyl group, and a vacant catalytic site (□) with the Ti(III) ion in the center of the octahedron.
A polymerized ligand, such as ethylene, occupies the vacant site:
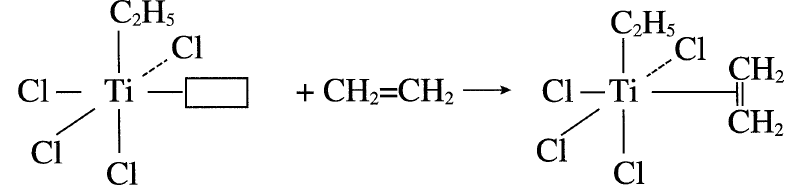
The next step is the cis insertion of the ethyl group, leaving a vacant site.
In another step, ethylene occupies the vacant site. This process continues until the propagating chain terminates:

When propylene is polymerized with free radicals or some ionic initiators, a mixture of three stereo-forms results (Figure 1.1). These forms are
Atactic—the methyl groups are randomly distributed. Isotactic—all methyl groups appear on one side of the polymer chain.
Syndiotactic—the methyl groups alternate regularly from one side to the other.
The isotactic form of propylene has better physical and mechanical properties than the three tactic form mixture (obtained from free radical polymerization). Isotactic polypropylene, in which all of the stereo cen- ters of the polymer are the same, is a crystalline thermoplastic.
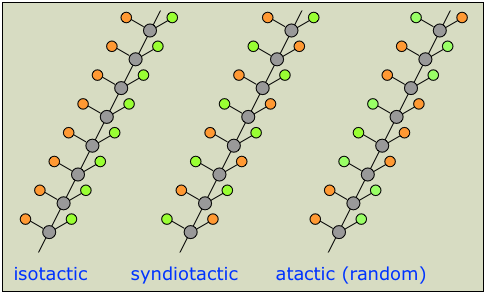
Figure 1.1. Propylene can undergo polymerization in three different ways to form atactic (a), syndiotactic (b), or isotactic polypropylene (c).
By contrast, atactic polypropylene, in which the stereo centers are arranged randomly, is an amorphous gum elastomer. Polypropylene consisting of blocks of atactic and isotactic stereo a sequence is rubbery. Polymerizing propylene with Ziegler-Natta catalyst produces mainly isotactic polypropylene. The Cosse-Arlman model explains the formation of the stereoregular type by describing the crystalline structure of αTiCl3 as a hexagonal close packing with anion vacancies. This structure allows for cis insertion. However, due to the difference in the steric requirements, one of the vacant sites available for the ligand to link with the titanium catalyst which has a greater affinity for the propagating polymer than the other site. Accordingly, the growing polymer returns rapidly back to that site as shown here:

The propagating polymer then terminates, producing an isotactic polypropylene. Linear polyethylene occurs whether the reaction takes place by insertion through this sequence or, as explained earlier, by ligand occupation of any available vacant site. This course, however, results in a syndiotactic polypropylene when propylene is the ligand.
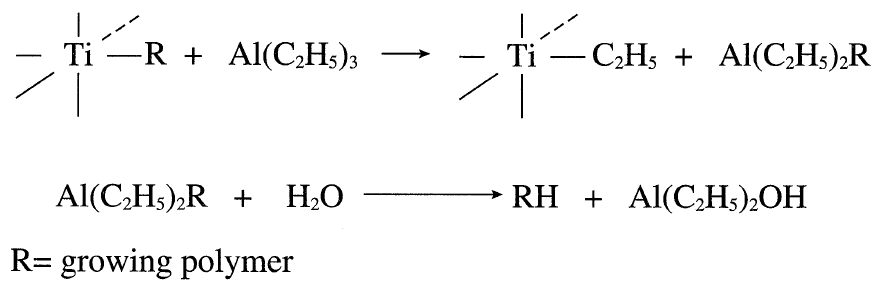
Adding hydrogen terminates the propagating polymer. The reaction between the polymer complex and the excess triethylaluminum also terminates the polymer. Treatment with alcohol or water releases the polymer:
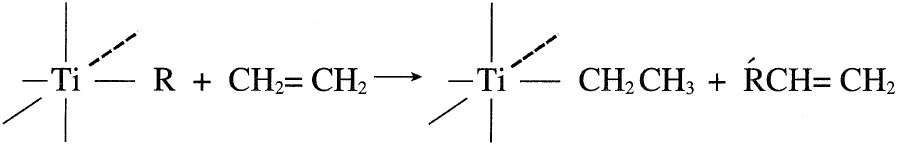
A chain transfer reaction between the monomer and the growing polymer produces an unsaturated polymer. This occurs when the concentration of the monomer is high compared to the catalyst. Using ethylene as the monomer, the termination reaction has this representation:
A new generation coordination catalysts are metallocenes. The chiral form of metallocene produces isotactic polypropylene, whereas the achiral form produces atactic polypropylene. As the ligands rotate, the catalyst produces alternating blocks of isotactic and atactic polymer much like a miniature sewing machine which switches back and forth between two different kinds of stitches.
 الاكثر قراءة في البترو كيمياويات
الاكثر قراءة في البترو كيمياويات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















