
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-4-2017
Date: 16-4-2017
Date: 19-3-2016
|
Dilution Problems
If a solution is diluted, the volume is increased and the concentration of solute decreased, but the number of moles of solute remains constant. Since C = n/V, n = CV, and we have a simple relation between the original molar concentration and volume and the final concentration and volume after dilution:
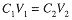
The subscripts here refer to the particular state of teh system, 1 to the initial state, and 2 to the final state
Example 3
Suppose that 25 mL of the 1.83 M NaCl solution is diluted to 100 mL. What is the final molar concentration of NaCl?
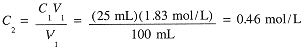



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|