


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-1-2022
Date: 16-1-2022
Date: 21-7-2016
|
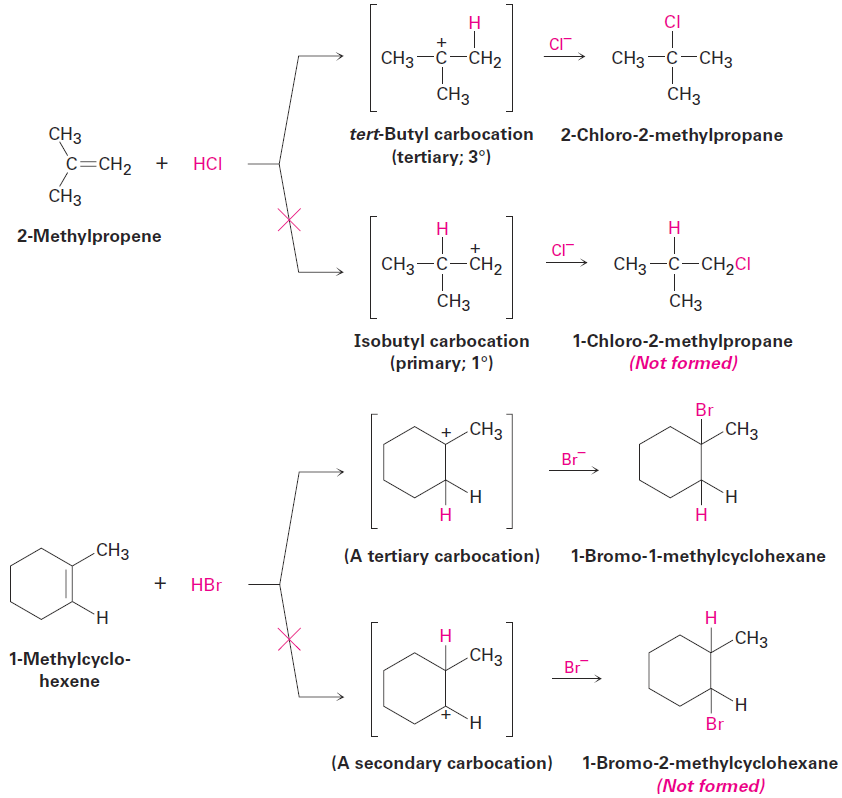
Markovnikov’s rule (restated)
In the addition of HX to an alkene, the more highly substituted carbocation is formed as the intermediate rather than the less highly substituted one. For example, addition of H+ to 2-methylpropene yields the intermediate tertiary carbocation rather than the alternative primary carbocation, and addition to 1-methylcyclohexene yields a tertiary cation rather than a secondary one. Why should this be?
q/ Predicting the Product of an Electrophilic Addition Reaction
What product would you expect from reaction of HCl with 1 ethylcyclopentene?
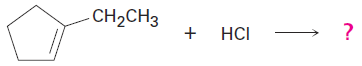
S t r a t e g y
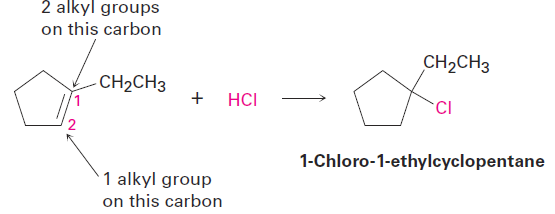
When solving a problem that asks you to predict a reaction product, begin by looking at the functional group(s) in the reactants and deciding what kind of reaction is likely to occur. In the present instance, the reactant is an alkene that will probably undergo an electrophilic addition reaction with HCl. Next, recall what you know about electrophilic addition reactions to predict the product. You know that electrophilic addition reactions follow Markovnikov’s rule, so H1 will add to the double-bond carbon that has one alkyl group (C2 on the ring) and the Cl will add to the double-bond carbon that has two alkyl groups (C1 on the ring).
S o l u t i o n
The expected product is 1-chloro-1-ethylcyclopentane.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|