


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-8-2016
Date: 12-4-2017
Date: 25-7-2016
|
Molarity in stoichiometry: Figuring out how much you need
The usefulness of the molarity concentration unit is readily apparent when dealing with reaction stoichiometry. For example, suppose that you want to know how many milliliters of 2.50 M sulfuric acid it takes to neutralize a solution containing 100.0 grams of sodium hydroxide. The first thing you must do is write the balanced chemical equation for the reaction:
H2SO4(aq) + 2 NaOH(aq) → 2 H2O(l) + Na2SO4(aq)
You know that you have to neutralize 100.0 grams of NaOH. You can convert the weight to moles (using the formula weight of NaOH, 40.00 g/mol) and then convert from moles of NaOH to moles of H2SO4. Then you can use the molarity of the acid solution to get the volume:
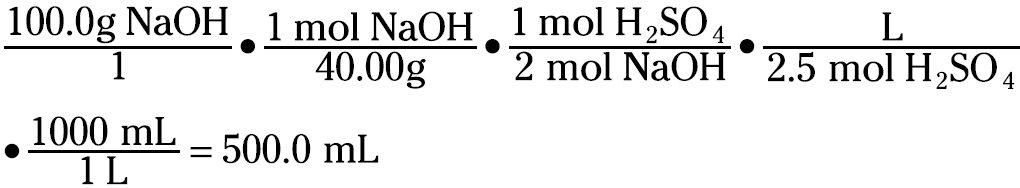
It takes 500.0 milliliters of the 2.50 M H2SO4 solution to completely react with the solution that contains 100 grams of NaOH.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|