


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Biomolecules Are Compounds of Carbon with a Variety of Functional Groups |
|
|
|
Read More
Date: 25-1-2017
Date: 11-9-2016
Date: 26-7-2016
|
Biomolecules Are Compounds of Carbon with a Variety of Functional Groups
The chemistry of living organisms is organized around carbon, which accounts for more than half the dry weight of cells. Carbon can form single bonds with hydroge atoms, and both single and double bonds with oxygen and nitrogen atoms (Fig. 1–1).
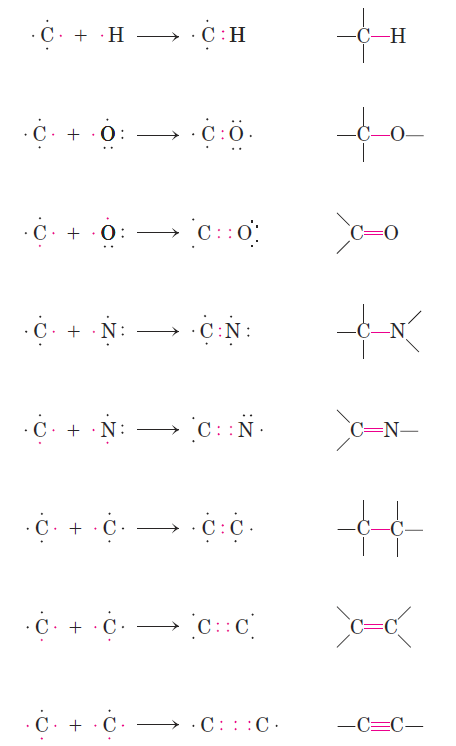
FIGURE 1–1 Versatility of carbon bonding. Carbon can form covalent single, double, and triple bonds (in red), particularly with other carbon atoms. Triple bonds are rare in biomolecules.
Of greatest significance in biology is the ability of carbon atoms to form very stable carbon–carbon single bonds. Each carbon atom can form single bonds with up to four other carbon atoms. Two carbon atoms also can share two (or three) electron pairs, thus forming double (or triple) bonds. The four single bonds that can be formed by a carbon atom are arranged tetrahedrally, with an angle of about 109.5_ between any two bonds (Fig. 1–2) and an average length of 0.154 nm. There is free rotation around each single bond, unless very large or highly charged groups are attached to both carbon atoms, in which case rotation may be restricted. A double bond is shorter (about 0.134 nm) and rigid and allows little rotation about its axis.
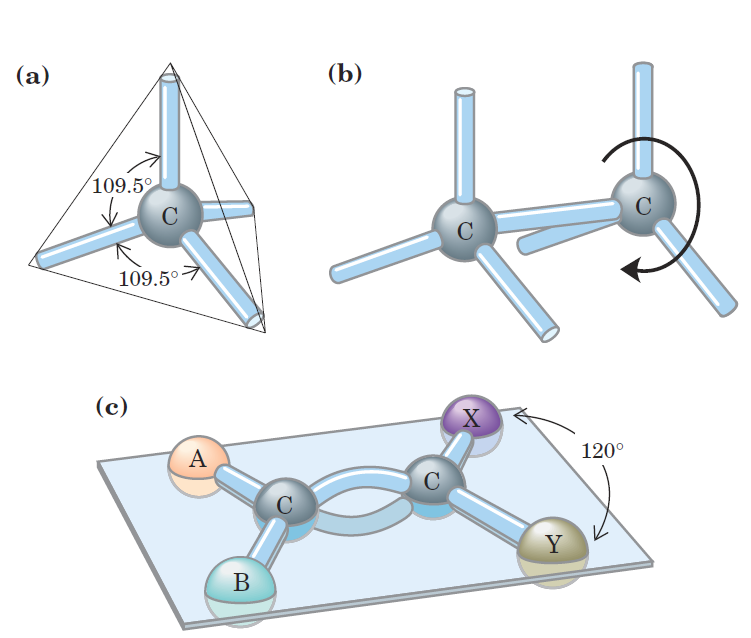
FIGURE 1–2 Geometry of carbon bonding. (a) Carbon atoms have a characteristic tetrahedral arrangement of their four single bonds. (b) Carbon–carbon single bonds have freedom of rotation, as shown for the compound ethane (CH3OCH3). (c) Double bonds are shorter and do not allow free rotation. The two doubly bonded carbons and the atoms designated A, B, X, and Y all lie in the same rigid plane.
Covalently linked carbon atoms in biomolecules can form linear chains, branched chains, and cyclic structures. To these carbon skeletons are added groups of other atoms, called functional groups, which confer specific chemical properties on the molecule. It seems likely that the bonding versatility of carbon was a major factor in the selection of carbon compounds for the molecular machinery of cells during the origin and evolution of living organisms. No other chemical element can form molecules of such widely different sizes and shapes or with such a variety of functional groups. Most biomolecules can be regarded as derivatives of hydrocarbons, with hydrogen atoms replaced by a variety of functional groups to yield different families of organic compounds. Typical of these are alcohols, which have one or more hydroxyl groups; amines, with amino groups; aldehydes and ketones, with carbonyl groups; and carboxylic acids, with carboxyl groups (Fig. 1–3).

FIGURE 1–3 Some common functional groups of biomolecules. In this figure and throughout the book, we use R to represent “any substituent.” It may be as simple as a hydrogen atom, but typically it is a carbon-containing moiety. When two or more substituents are shown in a molecule, we designate them R1, R2, and so forth.
Many biomolecules are polyfunctional, containing two or more different kinds of functional groups (Fig. 1–4), each with its own chemical characteristics and reactions. The chemical “personality” of a compound is determined by the chemistry of its functional groups and their disposition in three-dimensional space.

FIGURE 1–4 Several common functional groups in a single biomolecule. Acetyl-coenzyme A (often abbreviated as acetyl-CoA) is a carrier of acetyl groups in some enzymatic reactions.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|