


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 15-1-2017
Date: 30-9-2018
Date: 17-12-2020
|
Homogeneous catalysis: Offering an easier path
The second type of catalyst is a homogeneous catalyst — one that is in the same phase as the reactants. It provides an alternative mechanism, or reaction pathway, that has a lower activation energy than the original reaction. For an example, check out the decomposition reaction of hydrogen peroxide:
2H2O2(l) → 2 H2O(l) + O2(g)
This is a slow reaction, especially if you keep the hydrogen peroxide cool in a dark bottle. The hydrogen peroxide in that bottle in your medicine cabinet may take years to decompose. But if you put a little bit of a solution containing the ferric ion in the bottle, the reaction will be much faster, even though it’ll be a two-step mechanism instead of a one-step mechanism:
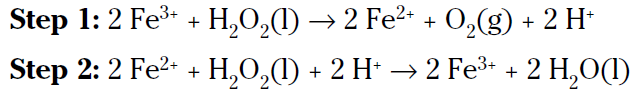
If you add the two preceding reactions together and cancel the species that are identical on both sides, you get the original, uncatalyzed reaction (species to be cancelled are bolded):
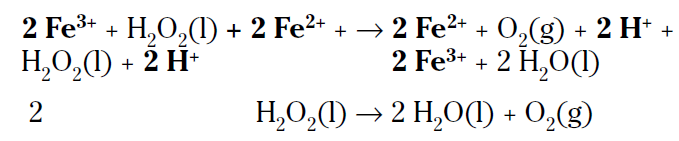
The ferric ion catalyst was changed in the first step and then changed back in the second step. This two-step catalyzed pathway has a lower activation energy and is faster.



|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|