
 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 4-9-2020
Date: 8-12-2020
Date: 1-1-2017
|
Alpha emission
An alpha particle is defined as a positively charged particle of a helium nucleus. This particle is composed of two protons and two neutrons, so it can be represented as a helium-4 atom. As an alpha particle breaks away from the nucleus of a radioactive atom, it has no electrons, so it has a +2 charge.
Therefore, it’s a positively charged particle of a helium nucleus. But electrons are basically free easy to lose and easy to gain. So normally, an alpha particle is shown with no charge because it very rapidly picks up two electrons and becomes a neutral helium atom instead of an ion.
Large, heavy elements, such as uranium and thorium, tend to undergo alpha emission. This decay mode relieves the nucleus of two units of positive charge (two protons) and four units of mass (two protons + two neutrons). What a process! Each time an alpha particle is emitted, four units of mass are lost. Radon-222 (Rn-222) is another alpha particle emitter, as the following equation shows:
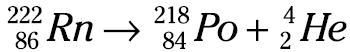
Here, radon-222 undergoes nuclear decay with the release of an alpha particle. The other remaining isotope must have a mass number of 218 (222 – 4) and an atomic number of 84 (86 – 2), which identifies the element as polonium (Po).



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
عبر مجلس عزاء.. العتبة العباسية المقدسة تستذكر فاجعة هدم قبور أئمة البقيع (عليهم السلام)
|
|
|