


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 17-10-2016
Date: 17-10-2016
Date: 27-10-2016
|
Evolution and The Origin of life
The species present today have evolved from those that existed in the past, which evolved from those that existed before them. It is appropriate now to consider how life arose and what it was like initially.
He most seriously considered hypothesis about the origin of life on Earth is that of chemosynthesis. Basically, the chemosynthetic hypothesis attempts to model the origin of life using only known chemical and physical processes, rejecting all traces of divine intervention. It was first proposed by the Russian scientist A. Oparin in 1924 and then by J. B. S. Haldane in England. They postulated that prior to the origin of life, the surface of Earth was different from the way it is now, and the chemicals present then could react spontaneously, producing more complex chemicals that could in turn continue to react. Over millions of years, reactions might produce all the molecules necessary for life, and these might aggregate into primitive proto-cells. From the proto-cells, natural selection would guide the evolution of true, living cells. Four conditions would have been necessary for the chemosynthetic origin of life: The primitive Earth would have to have had (1) the right inorganic chemicals, (2) appropriate energy sources, (3) a great deal of time, and (4) an absence of oxygen in its destructive molecular form, O2.
CONDITIONS ON EARTH PRIOR TO THE ORIGIN OF LIFE
Chemicals Present in the Atmosphere. Earth condensed from gases and dust about 4.6 billion years ago; it was initially hot and rocky and had an atmosphere composed mostly of hydrogen. Because hydrogen is such a light gas, most of this first atmosphere was lost into space. It was replaced by a second atmosphere produced by release of gases from the rock matrix composing Earth and from heavy bombardment by meteorites. Both sources would have provided gases such as hydrogen sulfide (H2S), ammonia (NH3), methane (CH4),and water. All these are found in volcanic gases and in meteorites that still strike Earth. Molecular oxygen was absent; it had already combined with other elements, resulting in compounds such as water and silicates. The early second atmosphere was a reducing atmosphere due to the lack of molecular oxygen and the presence of powerful reducing agents (Fig. 1).
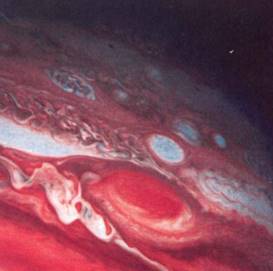
FIGURE 1:The outer planets, except for Pluto, are so massive that their gravity has retained their original hydrogen atmosphere. In addition, meteor bombardment and other activities have added ammonia, methane, and other components that make these atmospheres similar to Earth's early second atmosphere. Reactions in the atmosphere may be producing organic compounds. (NASA/Peter Arnold, Inc.)
Energy Sources. There must have been a complex chemistry in the early second atmosphere because it was exposed to powerful sources of energy. Foremost was intense UV and gamma radiation from the sun. These radiations have energetic quanta that knock electrons from atoms, creating highly reactive free radicals. Part of the ammonia would have decomposed to hydrogen and nitrogen, and some of the methane would have converted to carbon monoxide and carbon dioxide, increasing the complexity of the atmosphere.
Heat was another source of energy available to power reactions. One heat source was the coalescence of gas and dust to form Earth: As the particles fell toward the center of gravity, they accelerated and then collided, converting kinetic energy to heat. A second heat source was the radioactive decay of heavy elements like uranium and radium; this decay was extremely intense 4.5 billion years ago. Even today, enough radioactive decay remains to keep Earth's core molten. The chemicals present in the early second atmosphere were also dissolved in the ocean's water, and whenever they came into contact with hot or molten rock, endergonic reactions could proceed, resulting in the formation of more complex chemical compounds.
Electricity was abundant on a gigantic scale; much of Earth's water was initially suspended in the atmosphere because of the high temperature of the air and of the planet's surface. When sufficient planetary cooling occurred, rains fell, but the rainwater evaporated upon hitting the hot surface. The rainstorms must have been immense, lasting for thousands of years and generating tremendous amounts of lightning. As each lightning strike flashed through the atmosphere, it triggered more chemical reactions, and the re—ting products were washed downward by the rain: On the hot surface, they would have boiled, producing even more chemical reactions. After further planetary cooling, the surface temperature dropped to less than 100°C, and liquid water began to accumulate as streams, lakes, and oceans.
Volcanoes also produce lightning around their throats as they erupt (Fig. 2). Considering the immense amount of vulcanism that occurred, this lightning would have supplied a significant amount of energy. Also, volcanic lightning occurs through the clouds of venting gases where hydrogen sulfide, methane, ammonia, and water are most concentrated.

FIGURE 2 :Rapid movement of gases during a volcanic eruption generates the electrical potential necessary for lightning. Electrical discharges through the volcanic gases produce organic compounds. Mt. Kilauea, Hawaii. (E. R. Degginger).
Time Available for the Origin of Life. The time available for the chemosynthetic origin of life basically had no limits, simply because of the lack of free molecular oxygen. Without oxygen no agent was present to cause the breakdown and decomposition of the chemicals being created. If molecular oxygen had been present, the chemicals either would have not formed or would have oxidized soon after formation; without oxygen, they could accumulate for millions of years. The ocean of that time has been called a "dilute soup" or a 'pnmordail soup" containing water, salts, and numerous organic compounds that become increasingly complex as time went on. As much as 1.1 billion years may have elapsed between the time Earth solidified and life arose.
CHEMICALS PRODUCED CHEMOSYNTHETICALLY
After the writings of Oparin and Haldane, the first experimental tests of the chemosynthetic hypothesis were performed in 1953 by a graduate student, S. Miller, at the University of Chicago He constructed a container that had boiling water in the bottom and a reducing atmosphere in the top; electrodes discharged sparks into the gases, simulating lightning: As he water boiled, steam rose, mixed with the atmosphere and was reacted upon by the electrical sparks, then condensed and fell back into the water to be cycled again (Fig. 3). Miller let his first experiment cycle for a week and noticed that the solution had become dark from the accumulation of complex organic compounds that had formed. When he analyzed their composition, he found that many different substances woe present, including amino acids. Since then, this type of experiment has been performed numerous times, testing the effects of varying atmospheric compositions, using several types of energy sources, or including metal ions in the water. Virtually all the small molecules essential for life can be formed this way: amino acids, sugars, lipids, nitrogen bases, and so on.
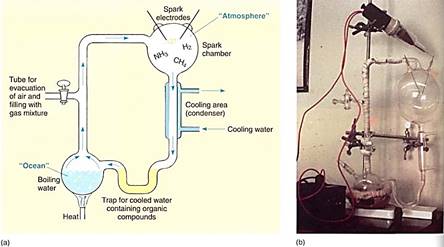
FIGURE 3: (a) Diagram and (b) photograph of apparatus used by Miller to show that the first steps in the chemosynthetic origin of life were possible. (Courtesy of Dr. Stanley Miller)
These experiments tell us what is theoretically possible; direct analysis of meteorites and lunar samples reveals what has actually happened in nonliving environments. Rock samples brought back from the moon by the Apollo astronauts contain various organic compounds, including amino acids. The interiors of meteorites, uncontaminated by the fall through the atmosphere or contact with soils, have contained alcohols, sugars, amino acids, and the nitrogenous bases that occur in nucleic acids. With regard to the formation of monomers, the chemosynthetic hypothesis represents a plausible model.
THE FORMATION OF POLYMERS
Monomers present in the early ocean had to polymerize if life were to arise, but polymerization required high concentrations of monomers. Given enough time, the oceans would have changed from a dilute soup to a concentrated one, but that probably was not necessary. Numerous mechanisms would have produced pools of highly concentrated reactants.
An obvious method of concentration is the formation of seaside pools at high tide that evaporate after the tide goes out. With intense sunlight the pools would have been warm, perhaps even hot, and polymerization reactions could occur. With the return of high tide, the polymers would be washed into the sea and accumulate. Monomers could also have accumulated when ponds and seaside pools froze; ice is relatively pure water and the monomers become increasingly concentrated in whatever water has not yet frozen. This might produce a class of polymers distinct from those formed by evaporation at high temperature.
Absorption by clay particles could have concentrated monomers, and clays are receiving great attention now. Because clay particles are tiny fragments of rock, they have a regular, crystalline surface; organic molecules adhere to them in a particular orientation, not simply at random. Thus, binding to a clay particle is similar to binding to an enzyme. Furthermore, the crystalline matrix of clay contains contaminating ions of iron, magnesium, calcium, phosphate, and other charged groups that are typically present at the active sites of enzymes. Considerable experimentation is being done to determine whether clays might have both concentrated monomers and acted as the first primitive catalysts.
AGGREGATION AND ORGANIZATION
The next step in the possible chemical evolution of life would have been the aggregation of chemical components into masses that had some organization and metabolism. Fatty, hydrophobic material would have accumulated automatically as oil slicks in quiet water or as droplets in agitated water (Fig. 4). Fatty acids would have occupied the outermost layer, accompanied by other molecules such as proteins that had a hydrophobic portion and a hydrophilic one. The interior may have been mostly hydrophobic, but proteins that had a hydrophobic exterior and hydrophilic interior would have added complexity. Aggregation of certain types of proteins would have resulted in large regions of hydrophilic sites.
These hrst aggregates would have formed basically at random, controlled only by relative solubility. If some of the proteins had some enzymatic activity by chance, the aggregate would have had some simple "metabolism"—perhaps the conversion of some molecule, absorbed from the sea, into another molecule; the aggregates would have been heterotrophs completely.
These aggregates are not postulated to have been alive or even to have been early stages of life, because at that point no means of storing genetic information existed. Without genetics, natural selection cannot occur. Countless numbers of these aggregates might have formed and disassociated throughout all the oceans over millions of years. Their existence may have had a significant effect on the chemistry of the oceans. Some may have provided appropriate conditions for certain proteins to be very active enzymes that might have produced quite complex products. However, others may have been able to degrade such products, so an equilibrium may have been established between the formation and destruction of ever more elaborate molecules and polymers.
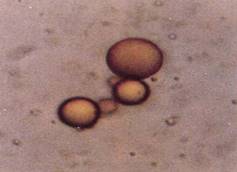
FIGURE 4: If dry proteins are heated mildly, they form a substance called proteinoid; if water is added, small round bodies called proteinoid microspheres are formed. They are round because of surface tension, just like plant protoplasts. They have an outer membrane that is differentially permeable; and some inner regions are aqueous, others hydrophobic. These cannot be considered living at all, but their differential permeability and their internal heterogeneity make them good sites for a primitive metabolism. (SEM by Steven Brooke, color copy arranged)
At some point, presumably an aggregate formed that did have a heritable information molecule able to direct the synthesis of products useful to the aggregate. By "useful" we mean that the product helped the aggregate persist longer, without being broken up by wave action or dissipating by diffusion. It may have helped the aggregate grow or even helped the information molecule replicate. With the presence of heredity, everything changed; any mutation that caused the production of a more efficient, more advantageous enzyme or structural protein provided a strong selective advantage and could be passed on to progeny aggregates. At present, attention is focusing on RNA as the first heritable information molecule. As the aggregate increased in size, perhaps largely by absorbing material from the ocean, its information molecule would replicate. If the aggregate divided into two by either wave action or surface tension and if each half contained an information molecule, reproduction occurred.
EARLY METABOLISM
The aggregates would have been complete heterotrophs, absorbing all material from the ocean and modifying only a few molecules. However, as aggregates continued to consume certain nutrients, scarcity occurred; any aggregate that could produce an enzyme capable of synthesizing the scarce molecule from an abundant one still available in the ocean would have had a strong selective advantage. With this, there would have been a metabolic pathway two steps long involving two enzymes (Fig. 5). Being able to synthesize a valuable, scarce molecule from an abundant, free one would have given that aggregate great advantage over the others; it would have had a more rapid metabolism and would have grown and reproduced more rapidly.
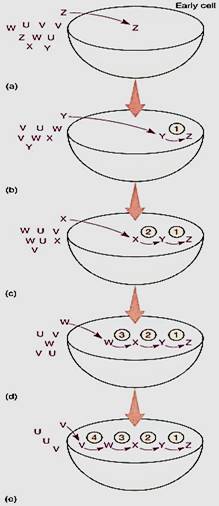
FIGURE 5 :We believe that many metabolic pathways evolved backward as raw materials in the environment became scarce. At present, the pathway for photosynthesis has gone as Hr as can, requiring only water and carbon dioxide.
As its abundance increased, it and its descendants would finally have become so numerous as to use the second chemical faster than it could be formed chemosynthetically, and scarcity would have occurred again. Natural selection would again favor any genetic system that could extend the metabolic pathway another step to include an abundant precursor. Recall that in photosynthesis in modern plants, the only two precursor molecules that need to be drawn from the environment are water and carbon dioxide—both are abundant and cheap. From them, metabolic pathways extend and ramify such that all carbon-containing compounds are derived from them. No preformed organic molecules are necessary.
Energy metabolism must have been important also, and glycolysis must have evolved early because it is present in virtually all organisms. The aggregates and first cells may have absorbed some ATP and generated more by fermentation with glycolysis for millions of years. At some point, electron transport systems and hydrogen ion pumping systems would have evolved. The first would have been powered by light, and an early form of photosynthesis would have evolved. Higher plant photosynthesis is elaborate, but much simpler types occur even today in certain photosynthetic bacteria. The evolution of photosynthesis would not have been as complex or as difficult as chloroplasts would lead us to believe. Oxidative electron transport, as occurs in mitochondria, could not have evolved because fee molecular oxygen still did not exist.
OXYGEN
The evolution of chlorophyll a and photosynthesis that liberates oxygen had two profound consequences: (1) it allowed the world to rust, and (2) it created conditions that selected for the evolution of aerobic respiration. Until that time, photosynthesis had involved the bacterial pigment bacteriochlorophylls, which liberates sulfur from hydrogen sulfide rather than oxygen from water (see Chapter 19). Earth retained its reducing atmosphere, and chemosynthesis of complex precursor molecules could continue. But once chlorophyll a evolved, the raw material for photosynthesis became water, and free molecular oxygen, O2, was released as a waste product. We know that this evolutionary step occurred 2.8 billion years ago because the oxygen rapidly combined with iron and formed ferric oxide—rust. Sedimentary rocks of this age contain a thick red layer of rust, indicating when oxygen-liberating photosynthesis arrived and how long the oxygen/iron reactions continued. Only after all the free iron in Earth's oceans had oxidized did oxygen finally begin to accumulate in the atmosphere. The atmosphere present today was derived from the early second atmosphere by this addition of oxygen from photosynthesis. It is an oxidizing atmosphere.
The period of rusting was critically important for all life because it kept the concentration of free oxygen very low. It can be difficult for us humans—obligate aerobic animals —to appreciate just how toxic free oxygen is; we need it to live, but our bodies have numerous mechanisms to keep it under control and to prevent it from reacting at random in our bodies. The same is true of plants, fungi, algae, and many bacteria. Had iron not been present, the concentration of free oxygen in water and air might have increased rapidly and killed everything; possibly there would have been no survivors. But with iron, oxygen concentration remained low for millions of years; it was an environmental danger but was not instantly, universally lethal. Any mutation that produced a mechanism that could detoxify oxygen had great selective advantage. Of course, the best way is to add two electrons to it and let it pick up two protons and turn into water, which is exactly what cytochrome oxidase does. This is the last electron carrier in the mitochondrion transport chain, and with its evolution, aerobic respiration became possible. Oxygen was transformed from a dangerous pollutant to a valuable resource. The evolution of aerobic respiration did not occur in mitochondria but rather in bacteria, and some species seem to retain very relictual, early versions.
The build-up of atmospheric oxygen had other important effects; under the influence of UV light, oxygen is transformed into ozone, O3. Ozone is extremely opaque to UV light and thus prevents most of it from penetrating deep into the atmosphere and reaching Earth's surface. This immediately removed UV light as an energy source and must have greatly slowed the chemosynthetic formation of organic molecules. Biological photosynthesis became the main means of bringing energy into the living world. The lack of UV radiation made the surface a safer place to live; UV radiation damages nucleic acids, and while the atmosphere lacked ozone, probably nothing lived near the top of the oceans or on land. With the ozone shield, organisms could move higher in the oceans and onto the mud of seashores. The transition to terrestrial life became possible.
THE PRESENCE OF LIFE
The chemosynthetic theory postulates a long series of slow, gradual transitions from completely inorganic compounds to living bacteria. At which stage can we say that life came into being? Can the aggregates be considered alive? This is a difficult question, because the theory delineates no absolute demarcation between living and nonliving objects (Fig 6). The chemistry of living creatures is more complex than that of nonliving objects, but it does not possess any unique properties. The physics of living and nonliving systems is identical. As so often in the living world, we are dealing with a continuum, not simply two mutually exclusive alternatives. To look for a dividing line would be simplistic; it is more important for us to understand the processes in their complexity.
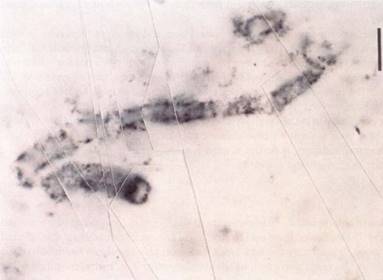
FIGURE 6 : The fossil remains of an early prokaryote that lived about 3.5 billion years ago. (Biological Photo Service).



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
المجمع العلمي يقيم ورشة تطويرية ودورة قرآنية في النجف والديوانية
|
|
|