


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-5-2017
Date: 20-1-2020
Date: 8-1-2020
|
Alkene Stereochemistry and the E,Z Designation
The cis–trans naming system used in the previous section works only with disubstituted alkenes—compounds that have two substituents other than hydrogen on the double bond. With trisubstituted and tetrasubstituted double bonds, a more general method is needed for describing double-bond geometry. (Trisubstituted means three substituents other than hydrogen on the double bond; tetrasubstituted means four substituents other than hydrogen.)
Let’s briefly review the sequence rules and then see how they’re used to specify doublebond geometry.
Rule 1
Considering each of the double-bond carbons separately, look at the two substituents attached and rank them according to the atomic number of the first atom in each. An atom with higher atomic number ranks higher than an atom with lower atomic number.
Rule 2
If a decision can’t be reached by ranking the first atoms in the two substituents, look at the second, third, or fourth atoms away from the double-bond until the first difference is found.
Rule 3
Multiple-bonded atoms are equivalent to the same number of singlebonded atoms.
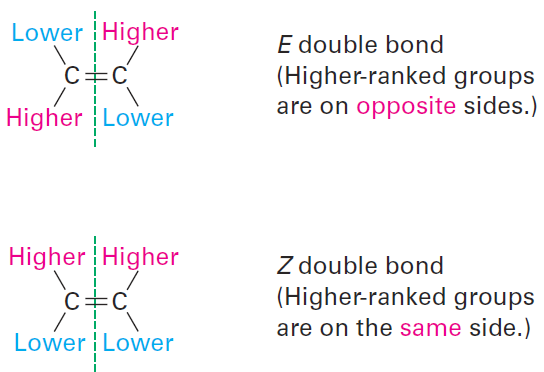
Once the two groups attached to each double-bonded carbon have been ranked as either higher or lower, look at the entire molecule. If the higherranked groups on each carbon are on the same side of the double bond, the alkene is said to have Z geometry, for the German zusammen, meaning “together.” If the higher-ranked groups are on opposite sides, the alkene has
E geometry, for the German entgegen, meaning “opposite.” (For a simple way to remember which is which, note that the groups are on “ze zame zide” in the Z isomer.)
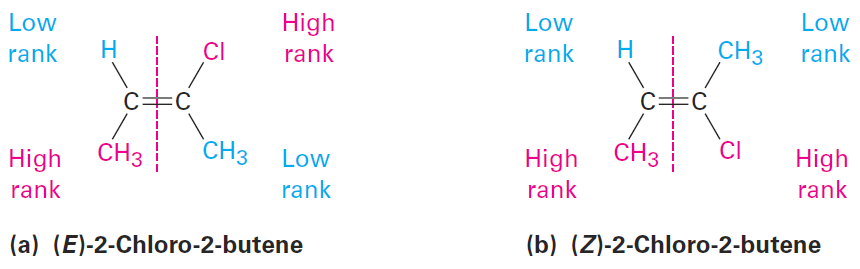
As an example, look at the following two isomers of 2-chloro-2-butene. Because chlorine has a higher atomic number than carbon, a-Cl substituent is ranked higher than a -CH3 group. Methyl is ranked higher than hydrogen, however, so isomer (a) is designated E because the higher-ranked groups are on opposite sides of the double bond. Isomer (b) has Z geometry because its higher-ranked groups are on ze zame zide of the double bond.
For further practice, work through each of the following examples to convince yourself that the assignments are correct:
Assigning E and Z Configurations to Substituted Alkenes
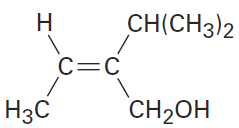
Assign E or Z configuration to the double bond in the following compound:
S t r a t e g y
Look at the two substituents connected to each double-bonded carbon, and determine their ranking using the Cahn–Ingold–Prelog rules. Then, check whether the two higher-ranked groups are on the same or opposite sides of the double bond.
S o l u t i o n
The left-hand carbon has -H and -CH3 substituents, of which -CH3 ranks higher by sequence rule 1. The right-hand carbon has -CH(CH3)2 and -CH2OH substituents, which are equivalent by rule 1. By rule 2, however, -CH2OH ranks higher than -CH(CH3)2 because the substituent -CH2OH has an oxygen as its highest second atom, but -CH(CH3)2 has a carbon as its highest second atom. The two higher-ranked groups are on the same side of the double bond, so we assign a Z configuration.




|
|
|
|
علامات بسيطة في جسدك قد تنذر بمرض "قاتل"
|
|
|
|
|
|
|
أول صور ثلاثية الأبعاد للغدة الزعترية البشرية
|
|
|
|
|
|
|
مكتبة أمّ البنين النسويّة تصدر العدد 212 من مجلّة رياض الزهراء (عليها السلام)
|
|
|