

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Resonance
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
p36
26-2-2016
3315
Resonance
Most substances can be represented unambiguously by the Kekulé line-bond structures we’ve been using up to this point, but an interesting problem sometimes arises. Look at the acetate ion, for instance. When we draw a line-bond structure for acetate, we need to show a double bond to one oxygen and a single bond to the other. But which oxygen is which? Should we draw a double bond to the “top” oxygen and a single bond to the “bottom” oxygen, or vice versa?
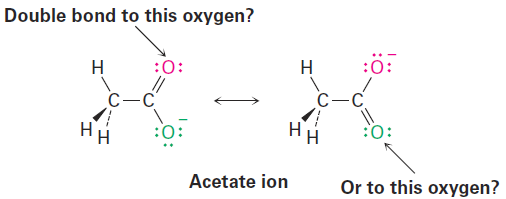
Although the two oxygen atoms in the acetate ion appear different in linebond structures, experiments show that they are equivalent. Both carbon– oxygen bonds, for example, are 127 pm in length, midway between the length of a typical C- O single bond (135 pm) and a typical C=O double bond (120 pm). In other words, neither of the two structures for acetate is correct by itself. The true structure is intermediate between the two, and an electrostatic potential map shows that both oxygen atoms share the negative charge and have equal electron densities (red).
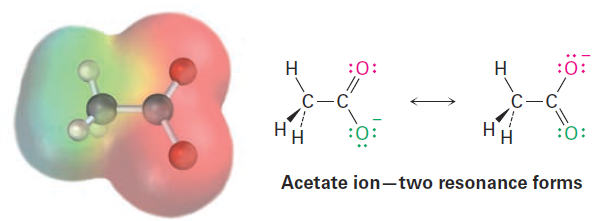
The two individual line-bond structures for acetate ion are called resonance forms, and their special resonance relationship is indicated by the double-headed arrow between them. The only difference between resonance forms is the placement of the and nonbonding valence electrons. The atoms themselves occupy exactly the same place in both resonance forms, the connections between atoms are the same, and the three-dimensional shapes of the resonance forms are the same.
A good way to think about resonance forms is to realize that a substance like the acetate ion is the same as any other. Acetate doesn’t jump back and forth between two resonance forms, spending part of the time looking like one and part of the time looking like the other. Rather, acetate has a single unchanging structure that we say is a resonance hybrid of the two individual forms and has characteristics of both. The only “problem” with acetate is that we can’t draw it accurately using a familiar line-bond structure—line-bond structures just don’t work well for resonance hybrids. The difficulty, however, is with the representation of acetate on paper, not with acetate itself. The six carbon–carbon bonds in aromatic compounds, such as benzene, are equivalent and that benzene is best represented as a hybrid of two resonance forms. Although each individual resonance form seems to imply that benzene has alternating single and double bonds, neither form is correct by itself. The true benzene structure is a hybrid of the two individual forms, and all six carbon–carbon bonds are equivalent. This symmetrical distribution of electrons around the molecule is evident in an electrostatic potential map.

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















