
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-7-2018
Date: 9-9-2019
Date: 13-9-2019
|
Double bonds
In contrast to a single (σ) bond where free rotation is generally assumed, rotation about a double bond is not a low energy process. The presence of a double bond may therefore lead to geometrical isomerism as is observed for N2F2. Each N atom carries a lone pair as well as forming one N__F single bond and an N=N double bond. Structures 1.35 and 1.36 show the trans- and cis-isomers† respectively of N2F2.
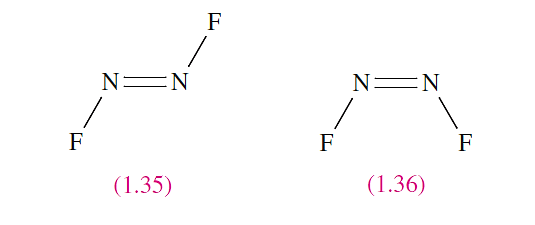




|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
خطيب صلاة العيد في كربلاء يطالب بتطبيق ما اوصى به الإمام علي ولده الحسن (ع) لمعالجة امراض اجتماعية خطرة استثمرها العدو لزرع التفرقة في المجتمع
|
|
|