


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 30-10-2020
Date: 2-12-2019
Date: 28-5-2017
|
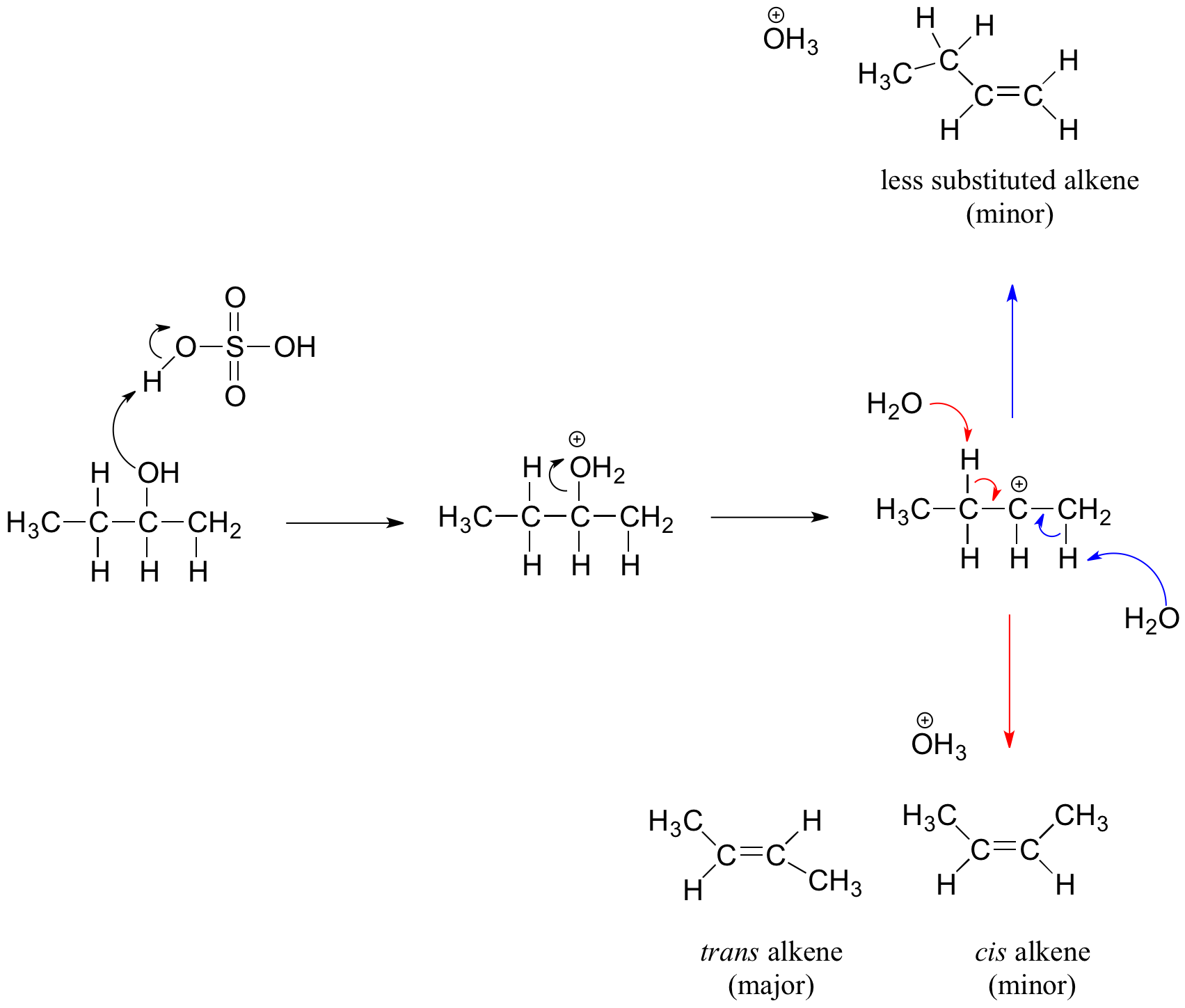
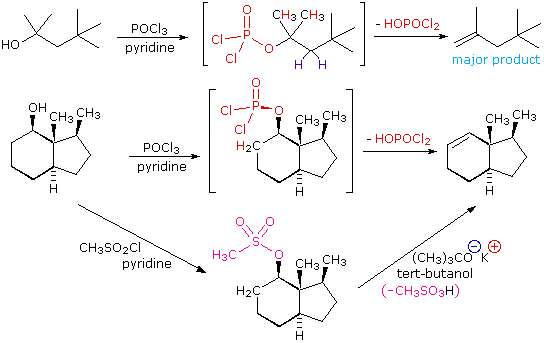
The E2 elimination of 3º-alcohols under relatively non-acidic conditions may be accomplished by treatment with phosphorous oxychloride (POCl3) in pyridine. This procedure is also effective with hindered 2º-alcohols, but for unhindered and 1º-alcohols an SN2 chloride ion substitution of the chlorophosphate intermediate competes with elimination. Examples of these and related reactions are given in the following figure. The first equation shows the dehydration of a 3º-alcohol. The predominance of the non-Zaitsev product (less substituted double bond) is presumed due to steric hindrance of the methylene group hydrogen atoms, which interferes with the approach of base at that site. The second example shows two elimination procedures applied to the same 2º-alcohol. The first uses the single step POCl3 method, which works well in this case because SN2 substitution is retarded by steric hindrance. The second method is another example in which an intermediate sulfonate ester confers halogen-like reactivity on an alcohol. In every case the anionic leaving group is the conjugate base of a strong acid.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|