
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-5-2019
Date: 6-7-2017
Date: 9-7-2020
|
Atomic number, mass number and isotopes
Nuclides, atomic number and mass number A nuclide is a particular type of atom and possesses a characteristic atomic number, Z, which is equal to the number of protons in the nucleus; because the atom is electrically neutral, Z also equals the number of electrons. The mass number, A, of a nuclide is the number of protons and neutrons in the nucleus. A shorthand method of showing the atomic number and mass number of a nuclide along with its symbol, E, is:

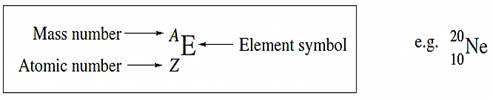
Atomic number = Z= number of protons in the nucleus = number of electrons
Mass number =A= number of protons number of neutrons
Number of neutrons=A- Z



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
بحضور الأمين العام للعتبة الحسينية.. قسم دار القرآن الكريم في العتبة الحسينية يكرم الكوادر المتطوعة في الختمة القرآنية الرمضانية
|
|
|