


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 28-12-2015
Date: 6-12-2015
Date: 14-4-2021
|
Lambda Repressor Dimers Bind Cooperatively to the Operator
KEY CONCEPTS
- Repressor binding to one operator increases the affinity for binding a second repressor dimer to the adjacent operator.
- The affinity is 10 times greater for OL1 and OR1 than other operators, so they are bound first.
- Cooperativity allows repressor to bind the OL2/OR2 sites at lower concentrations.
Each operator contains three repressor-binding sites. As can be seen in FIGURE 1, no two of the six individual repressorbinding sites are identical, but they all conform to a consensus sequence. The binding sites within each operator are separated by spacers of 3 to 7 bp that are rich in A-T base pairs. The sites at each operator are numbered so that OR consists of the series of binding sites OR1-OR2-OR3, whereas OL consists of the series OL1-OL2-OL3. In each case, site 1 lies closest to the start point for transcription in the promoter, and sites 2 and 3 lie farther upstream.
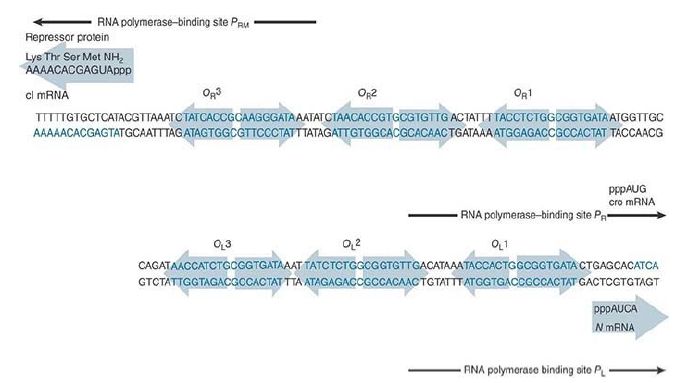
FIGURE 25.24 Each operator contains three repressor-binding sites and overlaps with the promoter at which RNA polymerase binds. The orientation of OL has been reversed from usual to facilitate comparison with OR .
Faced with the triplication of binding sites at each operator, how does the lambda repressor decide where to start binding? At each operator, site 1 has a greater affinity (roughly 10-fold) than the other sites for the lambda repressor. Thus, it always binds first to OL1 and OR1.
Lambda repressor binds to subsequent sites within each operator in a cooperative manner. The presence of a dimer at site 1 greatly increases the affinity with which a second dimer can bind to site 2. When both sites 1 and 2 are occupied, this interaction does not extend farther, to site 3. At the concentrations of the lambda repressor usually found in a lysogen, both sites 1 and 2 are filled at each operator, but site 3 is not occupied.
The C-terminal domain is responsible for the cooperative interaction between dimers, as well as for the dimer formation between subunits. FIGURE 2 shows that it involves both subunits of each dimer; that is, each subunit contacts its counterpart in the other dimer, forming a tetrameric structure.
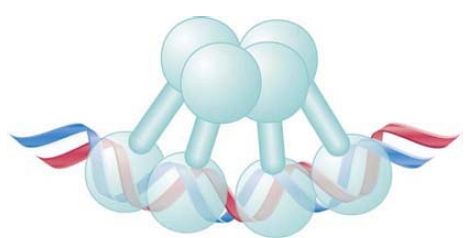
FIGURE 2. When two lambda repressor dimers bind cooperatively, each of the subunits of one dimer contacts a subunit in the other dimer.
A result of cooperative binding is the increase in effective affinity of repressor for the operator at physiological concentrations. This enables a lower concentration of repressor to achieve occupancy of the operator. This is an important consideration in a system in which release of repression has irreversible consequences. In an operon coding for metabolic enzymes, after all, failure to repress will merely allow unnecessary synthesis of enzymes. Failure to repress lambda prophage, however, will lead to induction of phage and lysis of the cell.
The sequences indicate that OL1 and OR1 lie more or less in the center of the RNA polymerase binding sites of PL and PR , respectively. Occupancy of OL1-OL2 and OR1-OR2 thus physically blocks access of RNA polymerase to the corresponding promoters.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|