


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 16-12-2015
Date: 16-4-2021
Date: 23-5-2016
|
Alpha-Lactalbumin
It requires patience to achieve quiet research. The investigation of properties of a-lactalbumin (a-LA) was not worthy of much notice until about 1965, especially from the standpoint of molecular biology. It is only a protein component of the complex enzyme “lactose synthase” and elucidation of its function had made little progress. However, the hidden connection to molecular biology was ferreted out in about 1967 and initiated the great explosion of activity in studying the molten globule (MG) conformation of proteins. These studies of the MG proteins opened a new area of structural biology connected to various physiological functions. Modern molecular biology sometimes requires the MG protein model to understand such physiological processes. The best-characterized MG is that formed by a-LA, and it can easily be prepared. a-LA is now one of the star proteins in molecular biology. The development of research into a-LA has been deeply affected by changing times.
One (a) of three peaks in the sedimentation velocity patterns of proteins in the non-casein fraction of skim milk was found responsible for a lactalbumin isolated from milk, which has subsequently been called a-lactalbumin. In the middle-1960s, it was confirmed that lactose is synthesized in the mammary gland UDP-galactose (UDP-Gal) and glucose (Glc) by an enzyme “lactose synthase,” which can be resolved into two fractions. One of them is a-LA, and the other is galactosyltransferase )GT). Alone in the Golgi apparatus, but with metal ions such as Mn2+, the latter catalyzes the transfer of Gal to GlcNAc on glycoproteins:
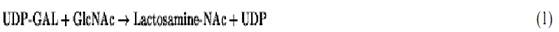
where NAc is the NAc is the N-acetyl group. a-LA is synthesized in the mammary gland during the lactation. It passes through the Golgi apparatus where it combines with GT and alters its substrate specificity to inhibit reaction 1. A decrease of three of magnitude in the Km for Glc facilitates the synthesis of lactose instead:

1. Structures
a-LA is normally a single polypeptide chain of 123 residues, four disulfide bonds, and a molecular weight of about 14,000 except for rat a-LA, which has 140 residues. About 1970, similarities were noted in the molecular weight and amino acid sequence between a-LA and hen egg white lysozyme (HEWL), a lytic enzyme. Subsequently, it was found that the exon-intron organization of the genes for both proteins are the same 1, 2. These suggested divergent evolution of the two proteins from a common ancestral gene, and a-LA was assumed to have a three-dimensional structure similar to that of HEWL, in spite of marked differences in their functions. X-ray crystallographic determination of the a-LA structure was not successful for a long time, but the conformations of both proteins in aqueous media were compared. Until 1980, significant differences in their physical behavior were observed:1) An intermediate conformation appears in the unfolding equilibrium of a-LA, but not of HEWL, at intermediate concentrations of guanidinium chloride (GdmCl) at a neutral pH, which is also stable at acid pH and is designated the “A-State”; 2) One Ca2+ ion is tightly bound to a-LA, but not to HEWL, and stabilizes the native (N) form. Removal of Ca2+ from a-LA induces the A-state near room temperature. Figure 1 shows a phase diagram of bovine a-LA for the N-, A-, and U- (unfolded) states in of GdmCl at 25°C, and the unfolding equilibrium has been explained in terms of the three states (3-5). Subsequently, it was shown that the kinetic intermediate of bovine a-LA captured at the early stage of refolding from U has properties similar to the equilibrium A-state. Later, it was reported that a number of proteins including HEWL assume the equilibrium and kinetic intermediate(s) similar to the A-state of a-LA in the molten globule (MG) form, and the MG was considered to be a third physical state of proteins (see Molten Globule). However, the conformational transition of the A to U of a-LA is nonco-operative, and then the folding of a-LA along the multipathway, which is apparently in the two-state type, is proposed (6-8). Also, recently, the kinetic intermediates of folding of a-LA with non-native secondary structures were shown, especially in the apo-form (9).
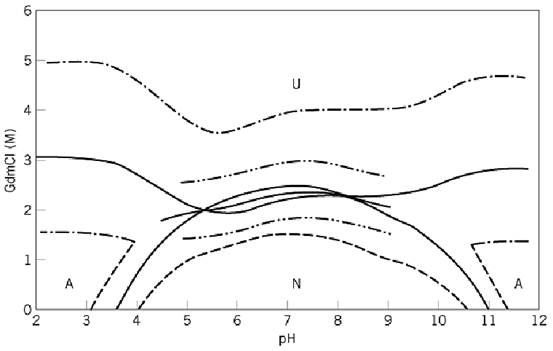
Figure 1. Phase diagram for the three states (N, A, and U) of bovine a-lactalbumin in a solution of GdmCl at any concentration and pH at 25°C.* The solid curves represent the pH and [GdmCl] where KeqNU Keq AU, or KeqNU is unit. Keq ij is for the conformational transition between i and j. The other curves represent the pH and [GdmCl] where Keq NA = 10 or 0.1 (---), Keq AU = 10 or 0.1 (-.-), and Keq NU = 10 or 0.1 (-. .-). *K. Kuwajima, Y. Ogawa, and S. Sugai (1983) J. Biochemistry (Tokyo) 89, 759–770.
Since 1987, the crystal structures of a-LA from various species (baboon, human, goat, guinea pig, and bovine) and their recombinant and mutant forms have been determined at resolutions of 1.15 to 2.4Å 10-15. The overall features of the authentic forms are similar to those of HEWL. Both structures are divided into two (a and b) subdomains by a deep cleft, the a-domain comprises residues 1 to 34 and 86 to 123, and the b-domain includes residues 35 to 85. In baboon a-domain contains four a-helices, A (residues 5 to 11), B (23 to 34), C (86 to 99), and D (105 to 109), plus single turns of 310-helices (12 to 16,101 to 104, and 115 to 119). The b-domain consists of a three-stranded antiparallel b-sheet (41 to 56), a 310-helix (76 to 82), and loop regions (Fig. 2). In a-LA from other species, however, the D-helix is replaced by a loop. The C-terminal region is flexible. The conformation and the ligand coordination of a Ca2+-binding site (Ka of 109 to 1010M–1 in the N-state) are observed at the interface of both the domains in all of a-LA. The Ca2+-binding site is composed of three Asp residues at 82, 87, and 88 and peptide carbonyl oxygens at residue 79 and 84 (the other two ligands are water molecules) and is in a slightly disordered, rigid pentagonal bipyramidal form (designated the “a-LA elbow”). The Asp residues are not found in the corresponding region of most lysozymes, but some have the Ca2+-binding site in an a-LA elbow (Table 1), which bind a Ca2+ ion in the N-state and are important for studying the evolution of the a-LA family. A Zn2+-binding site was also found in the human a-LA crystal, located at the entrance of the cleft between the two subdomains and coordinated with Glu 49, Glu 116, and two water molecules. Also, the Mn2+-and weak Ca2+-binding sites were also indicated. a-LA binds numerous other metal ions: Na+,K+,Ba2+,Mg2+,Co2+,Cu2+,Pb2+,Hg2+,Cd2+,Sr2+,Al3+,Tb3+,Eu3+,Sc3+, and Y3. However, elucidation of their locations and roles in lactose synthesis are awaited. Recently, binding of fatty acids to human a-LA is noted in connection with its function. The structures of a-LA determined by X-ray crystallography and by NMR in solution exhibit two hydrophobic clusters of aromatic residues: cluster I of Phe 31, His 32, Tyr 36, and Trp 118 and cluster II of Trp 26, Phe 53, Trp 60, and Trp 104 (16-19). These make up the cores in a-LA by combining with other hydrophobic parts in the a-domain.
Table 1. Comparison of “Equivalent Amino Acid Residues” in Some a-Lactalbumins and Lysozymes at the Calcium-Binding Sites

2. Molten Globule Intermediate
The molten globule of a-LA is compact (a radius of gyration only about 10% larger than that of the N-form) and has N-like secondary structures, but without the specific tertiary structural packing interactions. a-LA assumes the following stable MG forms in aqueous media: 1) An equilibrium unfolding state at intermediate concentrations of GdMCl or urea; 2 (The acid state; 3) A partially unfolded state obtained by removing of the bound Ca2+ at neutral pH and low salt concentrations, and 4) After partial reduction of the disulfide bonds. NMR and amide hydrogen exchange show heterogeneous structures of the MG, an a-domain relatively structured by hydrophobic interactions, and a more unfolded b-domain. Hydrophobic clusters I and II in the native form are somewhat rearranged locally, and some form of the hydrophobic core persists in the MG form and play as nuclei for the folding. The disulfide bond between Cys 6 and Cys 120 in native a-LA can be reduced extremely quickly by thiol agents. The free thiol groups in the three-disulfide intermediate (3SS) of a-LA are easily modified by iodoacetic acid or iodoacetoamide and generate carboxymethylated or carboxyamidomethylated 3SS a-LA, respectively. These trapped derivatives of bovine a-LA retain the N-form, with slight changes in the local conformation very near Cys 6 and Cys 120, but they assume the MG form in the absence of Ca2+ or at acid pH (20-25). A two-disulfide species and its trapped derivatives can also be obtained by subsequently reducing the 28–111 disulfide bond. The 2SS derivatives have partial MG characteristics. These disulfide intermediates and their derivatives are frequently used as models of partly unfolded proteins in the molecular biology because they are well characterized and easily prepared (26-28).
3. Functions
Lactose synthase (reaction 2) has long been studied to determine the binding sites of the saccharides and of metal ions and the interaction site between GT and a-LA. The structure of a-LA-GT complex has not been determined, but it has been shown by indirect methods such as chemical modification and site-directed mutagenesis that Ca2+ and some residues of a-LA adjacent to the cleft including Phe 31, His 32, Ala 106, and Leu 110 are crucial for the function of lactose synthase. The flexible C-terminal region, part of the hydrophobic clusters, and the cleft are essential for the lactose synthase function. The binding sites of a-LA on GT have been tentatively identified. The difference in function between a-LA and lysozyme has been explained by roles of some residues such Tyr 103 in a-LA. Mutations of six residues in the cleft of a-LA to the corresponding ones of lysozyme created a catalytic site of lysozyme in a-LA and hydrolyzed the glycoside bond, as chicken lysozyme (29).
It has been confirmed that molecular chaperones bind the protein and assist it folding in the cell. Physicochemical studies in vitro indicate that the chaperoninGroEL binds apo- or disulfide-reduced a-LA in the MG form, although it scarcely interacts with the N-state of a-LA (30-34). GroEL recognizes the hydrophobic surface exposed on the MG-form. a-LA bound to GroEL is also in the MG-form. a-LA and its disulfide-reduced forms in the MG interact with other chaperones and are frequently used as model substances for studies of protein folding in the cell. The insertion of soluble proteins into membranes and the conformations of the membrane-bound protein have been topics of interest (35-38). The insertion of a-LA into model membranes occurs under conditions that favor formation of the MG with its hydrophobic surface. The inserted a-LA is also in the MG-form, and its association with a lipid bilayer affects the chain mobility of the lipids.
Recently, some multimers of human a-LA prepared from its milk casein fraction were indicated to induce apoptosis in cancer cells, leukoma (L210), and lung carcinoma (A549) but not in healthy cells, although the monomeric human a-LA did not induce apoptosis in any cell (39-42). The active high oligomers are folding variants of human a-LA, to which fatty acids are bound, and assume the MG form. They were shown to accumulate in the nuclei of sensitive cells and to induce DNA fragmentation. The latter process requires Ca2+ ions. a-LA has important biological functions in addition to lactose synthesis.
a-LA is a protein of interesting structure, properties, and functions, and it will be noted in molecular biology in the future.
References
1. D. C. Phillips et al (1986) Biochem. Soc. Trans. 15, 737–744.
2. P. K. Qasba and S. Kumar (1997) Crit. Rev. Biochem. Mol. Biol. 32, 255–306.
3. K. Kuwajima et al (1976) J. Mol. Biol. 106, 359–373.
4. K. Kuwajima et al (1977) J. Mol. Biol. 114, 241–258.
5. M. Mizuguchi et al (2000) Proteins Struct. Funct. Genet. 38, 407–413.
6. W. Pfeil (1998) Proteins Struct. Funct. Genet. 30, 43–48.
7. M. Nozaka et al (1978) Biochemistry 17, 3753–3758.
8. M. Ikeguchi et al (1998) Protein Sci. 7, 1564–1574.
9. A. Troullier et al (2000) Nat. Struct. Biol. 7, 78–86.
10. R. Acharya et al (1989) J. Mol. Biol. 208, 99–127.
11. R. Acharya et al (1991) J. Mol. Biol. 221, 571–581.
12. R. Acharya et al (1996) Structure 4, 691–703.
13. R. Acharya et al (1998) Biochemistry 37, 4767–4772.
14. K. Harata et al (1999) J. Mol. Biol. 287, 347–358.
15. K. Harata et al (2000) J. Biol. Chem. 275, 37021–37029.
16. A. T. Alexandrescu et al (1992) Eur. J. Biochem. 210, 699–709.
17. A. T. Alexandrescu et al (1993) Biochemistry 32, 1707–1718.
18. C. M. Dobson et al (1999) J. Mol. Biol. 286, 1567–1580.
19. C. M. Dobson et al (1999) J. Mol. Biol. 288, 673–688.
20. J. J. Ewbank and T. E. Creighton (1991) Nature 350, 518–520.
21. J. J. Ewbank and T. E. Creighton (1993) Biochemistry 32, 3677–3707.
22. J. J. Ewbank and T. E. Creighton (1994) Biochemistry 33, 1534–1538.
23. P. S. Kim et al (1995) Trans. R. Soc. London B 348, 43–47.
24. P. S. Kim et al (1996) Biochemistry 35, 859–863.
25. D. F. Moriarty et al (2000) Biochim. Biophys. Acta 1476, 9–19.
26. K. Brew et al (1991) J. Biol. Chem. 266, 698–703.
27. K. Brew et al (1996) Biochemistry 35, 9710–9715.
28. K. Brew et al (1999) Protein Eng. 12, 581–587.
29. Y. Xue et al (2001) Proteins Struct. Funct. Genet. 42, 17–22.
30. M. K. Hayer et al (1994) EMBO J. 13, 3192–3202.
31. C. V. Robinson et al (1994) Nature 372, 646–651.
32. K. Kuwajima et al (1996) J. Mol. Biol. 258, 827–838.
33. K. Kuwajima et al (1996) J. Mol. Biol. 264, 643–649.
34. K. Kuwajima et al (1999) J. Mol. Biol. 293, 125–137.
35. S. Bunuelos and A. Muga (1995) J. Biol. Chem. 270, 29910–29915.
36. S. Bunuelos and A. Muga (1996) FEBS Lett. 386, 21–25.
37.S. Bunuelos and A. Muga (1996) Biochemistry 35, 3892–3898.
38. K. M. Cauthern et al (1996) Protein Sci. 5, 1349–1405.
39. M. Svanborg et al (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 8064–8068.
40. M. Svanborg et al (1999) J. Biol. Chem. 274, 6386–6396.
41. M. Svanborg et al (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 4221–4226.
42. M. Svanborg et al (2001) Eur. J. Biochem. 268, 186–191.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|