


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 18-10-2015
Date: 5-11-2020
Date: 21-10-2015
|
Control Mechanisms
The cell possesses an extraordinary array of enzymes; each specialized to carry out an important function in the cell. However, in many cases it is critical that the enzymes only be active at certain times and not others. For example, the digestive enzymes secreted by cells lining the stomach and intestine must only be active once they have been secreted and not before. If they were active prior to secretion they would degrade the proteins within the very cells that synthesized them. Or consider the enzymes that carry out the many activities of cell division. If these are not held in tight control, a cell will divide inappropriately and may become cancerous.
Thus, it is critical for the cell to be able to control the activities of many of its enzymes, and a number of intricate mechanisms have evolved to do just that. In most cases the activity of an enzyme is achieved via changes in its conformation, or shape, and the four most common ways of achieving this are regulation by small molecules; regulation by phosphorylation; regulation by protein-interactions; and regulation by proteolytic cleavage.
Regulation by Small Molecules
Regulation of an enzyme can occur by the binding of a small molecule to a site distant from the active site, which is the binding site for the enzyme’s substrate. This is called allosteric regulation (from the Greek allo, meaning “other,” and steric, meaning “site”). Because the small molecule does not bind the active site, it does not function by blocking access to the substrate. Instead, it acts by changing the conformation of the protein. A classic example of this occurs with an enzyme called aspartate transcarbamoylase (AT- Case) from the bacterium E. coli.
ATCase is the first enzyme in a series of enzymes whose end product is cytidine triphosphate (CTP), which is used to make ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). CTP has been found to bind to ATCase and inhibit its activity. The binding of CTP to ATCase changes the conformation of the active site such that the affinity for substrates is decreased by up to 90 percent. Thus, the buildup of CTP shuts the entire pathway off, thereby maintaining a fairly constant supply. This type of inhibition, in which the end product of a reaction inhibits its own synthesis, is called feedback inhibition and is a common regulatory mechanism in biologic pathways. In a more extreme case, the amino acid tryptophan goes so far as to inhibit the synthesis of the mRNAs encoding the biosynthetic enzymes that synthesize it. Thus, in that case, the enzymes themselves are not even synthesized until they are needed.
An important class of proteins regulated by small molecules is the G- proteins. They are called G-proteins because they bind and are activated by guanosine triphosphate (GTP). G-proteins have intrinsic GTPase activity, meaning they convert the bound GTP molecule to GDP (guanosine diphosphate). Typically, when GDP is bound, the conformation of the protein is such that the molecule is inactive. A protein called a GTP exchange factor (GEF) stimulates the exchange of GDP for GTP, thus reactivating the G- protein.
The ras protein is a G-protein found in a number of different organisms. Research has shown that ras activates proteins involved in cell growth and division. It was first discovered in a virus that causes tumors in mice. It causes tumors when it is mutated such that its GTPase activity is defective. This causes the protein to always be bound to GTP (instead of GDP) and hence it remains active. Thus, overactive ras causes uncontrolled cell proliferation and cancer. It has since been found that up to 15 percent of human cancers involve a mutation in ras that inhibits its GTPase activity, making it an important protein in human disease. The normal protein is only activated when stimulants outside the cell, such as growth factors, signal it to grow and proliferate. Following a short period of activity, inherent GTPase activity of the ras returns it to the inactive form.
Interestingly, in the fruit fly, Drosophila melanogaster, ras serves a different function. The structure and regulation of ras in D. melanogaster is similar to that in mammalian cells, but instead of participating in a pathway signaling cell proliferation, it is involved in a pathway leading to the differentiation of a certain type of cell in the eye, the photoreceptor cell. The regulation of protein activity by a GTP-GDP switch is apparently evolutionarily ancient and has been adapted to serve a variety of different cellular functions.
Regulation by Phosphorylation
Phosphorylation means the addition of a phosphate group, PO43-. Phosphorylation of certain amino acids in a protein can occur via the action of a group of proteins called kinases. Kinases are classified into two broad classes based on the amino acid they phosphorylate: the serine/threonine class and the tyrosine class. All three of these amino acids contain a hydroxyl (-OH) side chain, to which a phosphate group can be attached. Phosphorylation often occurs on more than one amino acid in a protein, and the result is a conformational change that affects the protein’s activity. A second class of proteins, called phosphatases, reverses the activity of kinases by removing the phosphates, returning the proteins to their original forms. Phosphatases are also categorized by their substrate specificities, either serine/threonine or tyrosine, however, a few have the ability to dephosphorylate all three amino acid side chains. In the example of regulation by phosphorylation described below, dephosphorylation leads to activation. This is not always the case, and equally as many proteins are activated by phosphorylation.
Nuclear factor of activated T cells (NFAT) is a protein regulated by phosphorylation. NFAT is a transcription factor that is found in the cytoplasm and is phosphorylated in resting cells. Dephosphorylation causes a conformational change that allows it to be transported to the nucleus, where it binds other transcription factors to activate gene transcription. NFAT was first found to activate genes in T cells (an immune cell); however, it has since been found in a number of other cell types as well.
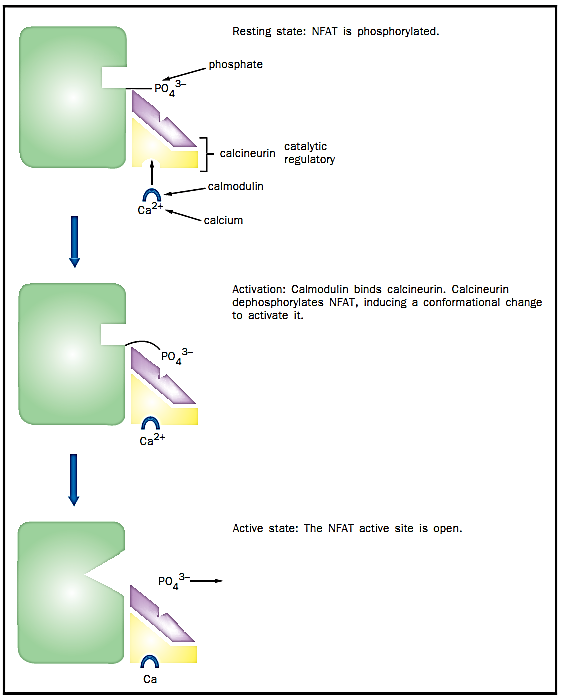
The phosphatase that dephosphorylates and activates NFAT is called calcineurin, which is of the serine/threonine class. Even when inactive, calcineurin is bound to NFAT, ensuring a rapid response upon activation. Stimulation by an inducer such as a viral infection or perhaps an organ transplant causes the activation of calcineurin, which then makes additional contacts with NFAT via its active site and dephosphorylates it. This activates NFAT, aiding the immune response by the T cell.
Regulation by Protein-Protein Interactions
Many enzymes are regulated by binding to another protein. For example, transcription factors, such as heat shock factor (HSF), can be activated by binding to other copies of itself (homomultimerization). In addition, inactivation of a protein can also occur by protein-protein interactions.

Phosphorylation or dephosphorylation causes a conformational change that affects the protein's activity.
Although HSF activation occurs by binding of identical molecules to one another, often regulation by protein-protein interactions occurs between different proteins. For example, calcineurin, the phosphatase that activates NFAT, is itself regulated by protein-protein interactions. Calcineurin is composed of two polypeptide subunits, one catalytic and one regulatory. The catalytic subunit contains the active site and is the part of the enzyme that dephosphorylates NFAT. The regulatory subunit binds the catalytic subunit and keeps it inactive by blocking the active site until a stimulus is detected.
The stimulus for calcineurin activation is increased cytoplasmic calcium levels. Calcium, together with a small protein called calmodulin, binds calcineurin, which results in the displacement of the regulatory subunit, exposing the active site and allowing it to dephosphorylate NFAT. Thus, calcineurin is an example of an enzyme that is regulated by both small molecules (calcium) and proteins (the regulatory subunit and calmodulin).
Another example of this occurs with a kinase called cAMP-dependent protein kinase, which in resting cells consists of a complex of two catalytic and two regulatory subunits. As with calcineurin, the regulatory subunits keep the kinase inactive until cyclic AMP (cAMP), a small molecule derived from adenosine monophosphate (AMP), binds and activates the kinase. In this case, however, the regulatory subunits completely dissociate from the catalytic subunits, rather than simply shifting their position as in calcineurin.
Regulation by Proteolytic Cleavage
A number of proteins are activated by proteolytic cleavage, that is, they are synthesized as a longer protein that is inactive and are later cleaved into a smaller, active form. The inactive precursor is called a zymogen, or proenzyme. For example, the hormone insulin is derived from proinsulin by proteolytic cleavage, as are many of the proteins involved in blood clotting. In addition, many digestive enzymes are activated by cleavage. Trypsinogen is a zymogen that upon cleavage becomes the digestive enzyme trypsin. Trypsinogen is made in the pancreas and secreted into the duodenum (the small intestine) where it is cleaved by an enzyme called enteropeptidase. The small amount of trypsin made by enteropeptidase then goes on to cleave other molecules of trypsinogen to make more trypsin as well as cleaving the other pancreatic zymogens into their active forms, and thus trypsin can be thought of as a master switch in the digestive process.
Unlike the other control mechanisms described above, proteolytic cleavage is irreversible, and thus once cleavage has occurred the enzyme cannot be returned to its inactive form. Thus, enzymes activated in this way may need to be turned off via other mechanisms. In the case of trypsin, there exists a small protein called pancreatic trypsin inhibitor that binds to trypsin’s active site and inhibits its activity. It binds the active site so tightly that even very harsh conditions used to dissociate proteins in the laboratory are ineffective at removing it. This extremely effective inhibitor has probably evolved to target trypsin because of its role as a master switch in the regulation of digestion in the small intestine. Once trypsin has been inactivated, proteolytic soon ceases, and digestion comes to a halt.
References
Cooper, Geoffrey. “Protein Synthesis, Processing and Regulation.” In The Cell: A Molecular Approach. Sunderland, MA: Sinauer Associates, Inc., 1997.
Stryer, Lubert. “Regulatory Strategies.” In Biochemistry, 4th ed. New York: W. H. Freeman and Company, 1995.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|