


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيويةAmino Acid
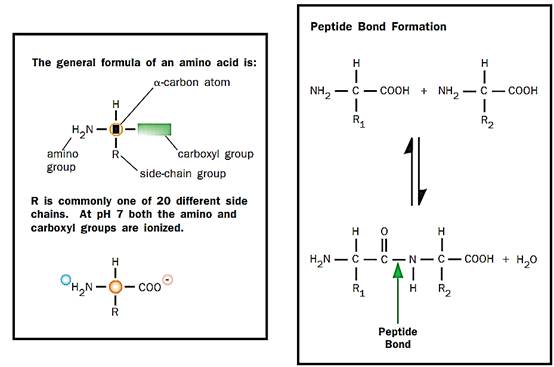
Amino acids are molecules that have both an amino group (-NH2) and a carboxylic acid group (-COOH), hence the name. The most common amino acids are the a-amino acids, the building blocks of proteins. These have the amino group, the carboxylic acid group, hydrogen, and a characteristic side chain all attached to one carbon atom, designated the a-carbon. Each type of a-amino acid has a unique side chain that determines its properties and its role in proteins. The side chains (or “R” groups) can range from a hydrogen atom, as in glycine, to the more complicated side chains of tryptophan or arginine.
The a-carbon atom has four different groups attached to it arranged at the points of a tetrahedron. This arrangement is asymmetric and can occur in two different forms, or enantiomers, that are related to each other in the same way as an object and its image in a mirror. These two enantiomers are called L and D. Only L-amino acids occur in proteins made by living systems. D-amino acids and amino acids other than a-amino acids occur in biological systems but are not incorporated into proteins.
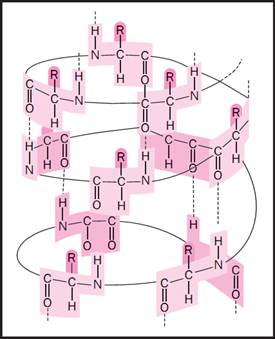
Amino acids link together to form alpha-helices and other fundamental structures, which interact to give proteins their ultimate three-dimensional shape.
Many organisms can synthesize all of the amino acids they require from compounds present in the metabolic pathways they use for energy production. Humans, however, are not able to synthesize all of the necessary amino acids, and a number of them must be obtained from the diet.
The major use of amino acids is to construct proteins. A protein is a linear chain of amino acids linked together by peptide bonds. A peptide bond is formed when the amino group attached to the a-carbon of one amino acid is joined to the carboxyl group of a second amino acid with the elimination of water. The side chain of each amino acid residue protrudes from the polypeptide backbone. The sequence of amino acids in the chain is determined by the deoxyribonucleic acid (DNA) sequence of the gene that codes for that protein.
The three-dimensional structure and the properties of a specific protein, and therefore its biological role, are determined by the sequence of amino acid side chains.
In proteins, acidic amino acid side chains are negatively charged, and basic ones are positively charged. The polar and charged amino acids are hydrophilic, meaning they like to interact with water (or are water-loving). The nonpolar, aromatic, and sulfur-containing amino acid side chains prefer to interact with themselves or each other (they are hydrophobic or water-avoiding).
A protein folds so that nonpolar side chains tend to be buried within the protein while polar and charged side chains tend to be exposed to the water around the protein. The biological function of a protein is generally highly dependent on its three-dimensional structure.
References
Alberts, Bruce, et al. Molecular Biology of the Cell, 4th ed. New York: Garland Publishing, 2000.
Stryer, Lubert. Biochemistry, 4th ed. New York: W. H. Freeman and Company, 1995.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|