


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 9-6-2021
Date: 29-12-2015
Date: 24-5-2021
|
The TCR Functions in Conjunction with the MHC
KEY CONCEPTS
- The TCR recognizes a short peptide set in the groove of a major histocompatibility complex (MHC) molecule on the surface of an antigen-presenting cell (APC).
- The recombination process to generate functional TCR chains is intrinsic to the development of T cells.
- The TCR is associated with the CD3 complex that is involved in transducing TCR signals from the cell surface to the nucleus.
T cells expressing TCRαβ comprise subtypes that have a variety of functions related to interactions with other cells of the immune system. CTLs possess the ability to lyse a target cell. Th cells help the activation/generation of CTLs or aid in the differentiation of B cells into antibody-producing cells.
The BCR/antibody and the TCR differ in their modalities of interaction with their ligands. A BCR/antibody recognizes a small area (epitope) within the antigen, which can be composed of a linear sequence (six to eight amino acids) identifying a linear determinant or a cluster of amino acids brought together by the three-dimensional structure of the antigen (conformation determinant). A TCR binds a peptide derived from the antigen upon processing by an APC. The peptide is generated when the proteasome degrades the antigen protein within the APC. It is “presented” to the T cell by the APC in the context of an MHC protein, in a groove on the surface of the MHC. Thus, the T cell simultaneously recognizes the peptide and an MHC protein carried by the APC. Both Th cells and CTLs recognize the antigen in this fashion, but with different requirements; that is, they recognize peptides of different sizes and as presented in conjunction with different types of MHC proteins . Th cells recognize peptide antigens, 13 to 20 amino acids long, presented by MHC class II proteins, whereas CTLs recognize peptide antigens, 8 to 10 amino acids long, presented by MHC class I proteins. The TCRαβ provides the structural correlate for the helper Th cell function and for the CTL function. In both cases, TCRαβ recognizes both the antigenic peptide and the self-MHC protein. A given TCR has specificity for a particular MHC, as well as for the associated antigen peptide. The basis for this dual recognition capacity is one of the most interesting structural features of the TCRαβ.
Recombination to generate functional TCR chains is linked to the development of the T lymphocyte (FIGURE 1). The first stage consists in rearrangement to form an active TCRβ chain. This binds a nonrearranging surrogate TCRα chain, which is called pre-TCRα. At this stage, the lymphocyte has not yet expressed either CD4 or CD8 on the surface. The pre-TCR heterodimer then associates with the CD3 signaling complex. Signaling from the complex triggers several rounds of cell division, during which TCRα chains are rearranged, and the CD4 and CD8 genes are turned on so that the lymphocyte transitions from CD4- CD8- , or double-negative (DN), thymocyte to CD4+ CD8+ , or double-positive (DP), thymocyte.
TCRα chain rearrangement continues in the DP thymocytes. The maturation process continues through both positive selection (for mature TCR complexes able to bind a self-ligand with moderate affinity) and negative selection (against complexes that interact with self-ligands at high affinity). Both positive and negative selection involve interaction with MHC proteins. DP thymocytes either die within 3 to 4 days or become mature lymphocytes as the result of the selection process. The surface TCRαβ heterodimer becomes cross-linked on the surface during positive selection, which rescues the thymocyte from apoptosis (nonnecrotic cell death). If thymocytes survive the subsequent negative selection, they give rise to the separate T lymphocyte subsets, CD4+ CD8- and CD4- CD8+ cells.
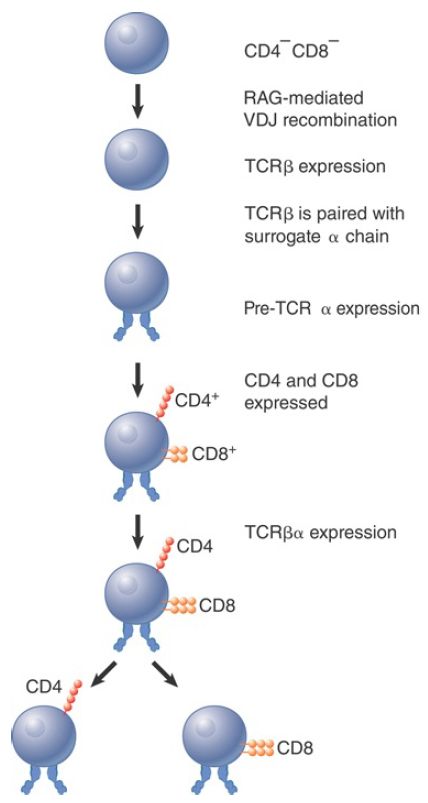
FIGURE 1. T cell development proceeds through sequential stages.
The TCR is associated with the CD3 complex of proteins, which are involved in transmitting a signal from the surface of the cell to the nucleus when the TCR is activated by binding of antigen (FIGURE 2). The interaction of the TCR variable regions with antigen causes the ζ chain of the CD3 complex to signal T cell activation, in a fashion comparable to the BCR Igα and Igβ complex signaling B cell activation.

FIGURE 2.The two chains of the T cell receptor (TCR) associate with the polypeptides of the CD3 complex. The variable regions of the TCR are exposed on the cell surface. The cytoplasmic domains of the ζ chains of CD3 provide the effector function.
Considerable diversity is required in both recognition of a foreign antigen, which requires the ability to respond to novel structures, and recognition of the MHC protein, which is restricted to one of the many different MHC proteins encoded in the genome. T cells and CTLs rely upon different classes of MHC proteins; however, they use the same pool of TCRα and TCRβ or TCRγ and TCRδ gene segments to assemble their TCRs. Even allowing for the introduction of additional variation during the TCR recombination process, the number of different TCRs generated is relatively limited, but nevertheless sufficient to satisfy the diversity demands imposed by the variety of TCR ligands. This is made possible by the relatively low binding affinity requirements by the TCRpeptide/ MHC interaction, which allows for one TCR to interact with multiple different ligands sharing some similarities.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|