


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 5-5-2021
Date: 28-3-2021
Date: 31-5-2021
|
Globin Clusters Arise by Duplication and Divergence
KEY CONCEPTS
- All globin genes are descended by duplication and mutation from an ancestral gene that had three exons.
-The ancestral gene gave rise to myoglobin, leghemoglobin, and α- and β-globins.
-The α- and β-globin genes separated in the period of early vertebrate evolution, after which duplications generated the individual clusters of separate α- and β-like genes.
-When a gene has been inactivated by mutation, it can accumulate further mutations and become a pseudogene (ψ), which is homologous to the functional gene(s) but has no functional role (or at least has lost its original function).
The most common type of gene duplication generates a second copy of the gene close to the first copy. In some cases, the copies remain associated and further duplication can generate a cluster of related genes. The best characterized example of a gene cluster is that of the globin genes, which constitute an ancient gene family fulfilling a function that is central to animals: the transport of oxygen.
The major constituent of the vertebrate red blood cell is the globin tetramer, which is associated with its heme (iron-binding) group in the form of hemoglobin. Functional globin genes in all species have the same general structure: They are divided into three exons.
Researchers conclude that all globin genes have evolved from a single ancestral gene, and by tracing the history of individual globin genes within and between species we can learn about the mechanisms involved in the evolution of gene families.
In red blood cells of adult mammals, the globin tetramer consists of two identical α chains and two identical β chains. Embryonic red blood cells contain hemoglobin tetramers that are different from the adult form. Each tetramer contains two identical α-like chains and two identical β-like chains, each of which is related to the adult polypeptide and is later replaced by it in the adult form of the protein. This is an example of developmental control, in which different genes are successively switched on and off to provide alternative products that fulfill the same function at different times.
The division of globin chains into α-like and β-like reflects the organization of the genes. Each type of globin is encoded by genes organized into a single cluster. The structures of the two clusters in the primate genome are illustrated in FIGURE 1. Pseudogenes are indicated by the symbol ψ.
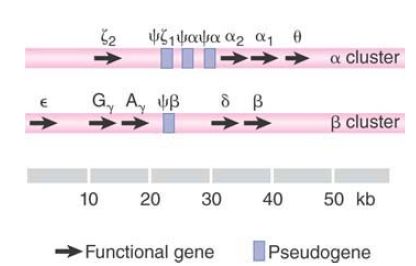
FIGURE 1 Each of the α-like and β-like globin gene families is organized into a single cluster, which includes functional genes and pseudogenes (ψ).
Stretching over 50 kb, the β cluster contains 5 functional genes (ε, two γ, δ, and β) and one nonfunctional pseudogene (ψβ). The two γ genes differ in their coding sequence in only one amino acid: The G variant has glycine at position 136, whereas the A variant has alanine.
The more compact α cluster extends over 28 kb and includes one functional ζ gene, one nonfunctional ζ pseudogene, two α genes, two nonfunctional α pseudogenes, and the θ gene of unknown function. The two α genes encode the same protein. Two (or more) identical genes present on the same chromosome are described as nonallelic genes.
The details of the relationship between embryonic and adult hemoglobins vary with the species. The human pathway has three stages: embryonic, fetal, and adult. The distinction between embryonic and adult is common to mammals, but the number of preadult stages varies. In humans, ξ and α are the two α-like chains. The β-like chains are γ, δ, and β. FIGURE 2 shows how the chains are expressed at different stages of development. There is also tissue-specific expression associated with the developmental expression: Embryonic hemoglobin genes are expressed in the yolk sac, fetal genes are expressed in the liver, and adult genes are expressed in bone marrow.
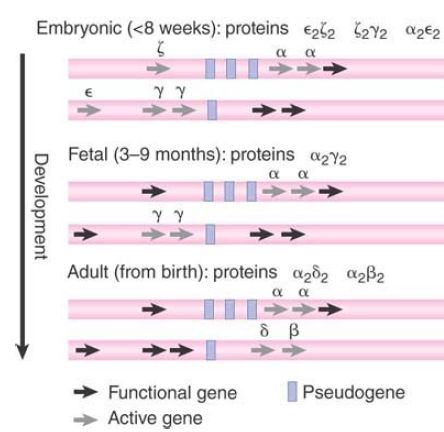
FIGURE 2. Different hemoglobin genes are expressed during embryonic, fetal, and adult periods of human development.
In the human pathway, ζ is the first α-like chain to be expressed,but it is soon replaced by α. In the β-pathway, ε and γ are expressed first, with δ and β replacing them later. In adults, the
α β form provides 97% of the hemoglobin, α2 δ2 provides about 2%, and about 1% is provided by persistence of the fetal form α 2γ 2.
What is the significance of the differences between embryonic and adult globins? The embryonic and fetal forms have a higher affinity for oxygen, which is necessary to obtain oxygen from the mother’s blood. This helps to explain why there is no direct equivalent (although there is temporal expression of globins) in, for example, the chicken, for which the embryonic stages occur outside the mother’s body (i.e., within the egg).
Functional genes are defined by their transcription to RNA and ultimately (for protein-coding genes) by the polypeptides they encode. Pseudogenes are defined as having lost their ability to
produce functional versions of polypeptides they originally encoded.
The reasons for their inactivity vary: The deficiencies might be in transcription, translation, or both. A similar general organization is found in all vertebrate globin gene clusters, but details of the types, numbers, and order of genes all vary, as illustrated in FIGURE 3. Each cluster contains both embryonic and adult genes. The total lengths of the clusters vary widely. The longest known cluster is found in the goat genome, where a basic cluster of four genes has been duplicated twice. The distribution of functional genes and pseudogenes differs in each case, illustrating the random nature of the evolution of one copy of a duplicated gene to a pseudogene.
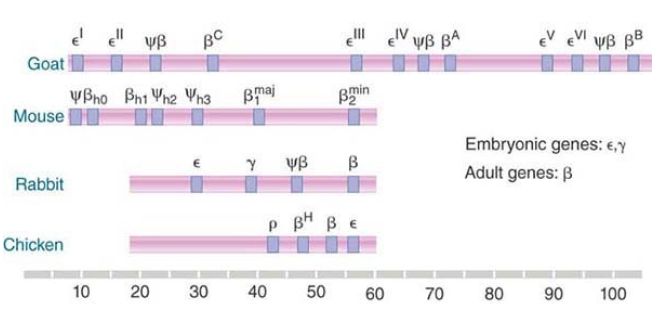
FIGURE 3. Clusters of β-globin genes and pseudogenes are found in vertebrates. Seven mouse genes include two early embryonic genes, one late embryonic gene, two adult genes, and two pseudogenes. Rabbits and chickens each have four genes.
The characterization of these gene clusters makes an important general point. There can be more members of a gene family, both functional and nonfunctional, than we would suspect on the basis of protein analysis. The extra functional genes might represent duplicates that encode identical polypeptides, or they might be related to—but different from—known proteins (and presumably expressed only briefly or in low amounts).
With regard to the question of how much DNA is needed to encode a particular function, we see that encoding the β-like globins requires a range of 20 to 120 kb in different mammals. This is much greater than we would expect just from scrutinizing the known β-globin proteins or even from considering the individual genes.
However, clusters of this type are not common; most genes are found as individual loci. From the organization of globin genes in a variety of species, we should be able to trace the evolution of present globin gene clusters from a single ancestral globin gene.
The leghemoglobin gene of plants, which is related to the globin genes, might provide some clues about the ancestral form, though of course the modern leghemoglobin gene has evolved for just as long as the animal globin genes. (Leghemoglobin is an oxygen carrier found in the nitrogen-fixing root nodules of legumes.) The furthest back that we can trace a true globin gene is to the sequence of the single chain of mammalian myoglobin, which diverged from the globin lineage about 800 million years ago in the ancestors of vertebrates. The myoglobin gene has the same organization as globin genes, so we can take the three-exon structure to represent that of their common ancestor.
Some members of the class Chondrichthyes (cartilaginous fish) have only a single type of globin chain, so they must have diverged from the lineage of other vertebrates before the ancestral globin gene was duplicated to give rise to the α and β variants. This appears to have occurred about 500 million years ago, during the evolution of the Osteichthyes (bony fish).
The next stage of globin evolution is represented by the state of the globin genes in the amphibian Xenopus laevis, which has two globin clusters. However, each cluster contains both α and β genes, of both larval and adult types. Therefore, the cluster must have evolved by duplication of a linked α–β pair, followed by divergence between the individual copies. Later, the entire cluster was duplicated.
The amphibians separated from the reptilian/mammalian/avian line about 350 million years ago, so the separation of the α- and β- globin genes must have resulted from a transposition in the reptilian/mammalian/avian forerunner after this time. This probably occurred in the period of early tetrapod evolution. There are separate clusters for α- and β-globins in both birds and mammals; therefore the α and β genes must have been physically separated before the mammals and birds diverged from their common ancestor, an event estimated to have occurred about 270 million years ago. Evolutionary changes have taken place within the separate α and β clusters in more recent times, as we saw from the description of the divergence of the individual genes in the section A Constant Rate of Sequence Divergence Is a Molecular Clock .



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|