


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-1-2019
Date: 31-8-2020
Date: 1-1-2017
|
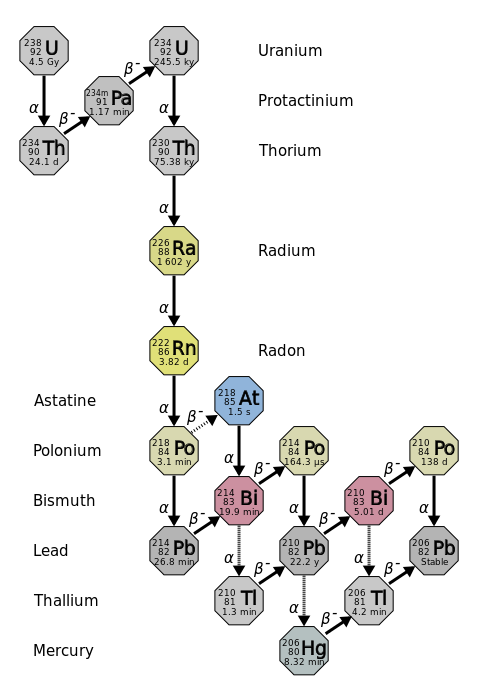
The decay of a radioactive nucleus is a move toward becoming stable. Often, a radioactive nucleus cannot reach a stable state through a single decay. In such cases, a series of decays will occur until a stable nucleus is formed. The decay of U
-238 is an example of this. The U-238 decay series starts with U-238 and goes through fourteen separate decays to finally reach a stable nucleus, Pb-206 (Figure1). There are similar decay series for U-235 and Th-232. The U-235 series ends with Pb-207 and the Th-232 series ends with Pb-208.
.svg.png?revision=1)
Figure1: Uranium-238 Decay chain. (CC-BY-3.0 Tosaka)
Several of the radioactive nuclei that are found in nature are present there because they are produced in one of the radioactive decay series. For example, there may have been radon on the earth at the time of its formation, but that original radon would have all decayed by this time. The radon that is present now is present because it was formed in a decay series (mostly by U-238).



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تدعو جامعة ديالى للمشاركة في حفل التخرج المركزي الخامس
|
|
|