


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Preparing Carboxylic Acids From nitriles and organometallic intermediates |
|
|
|
Read More
Date: 24-9-2020
Date: 14-10-2019
Date: 3-11-2019
|
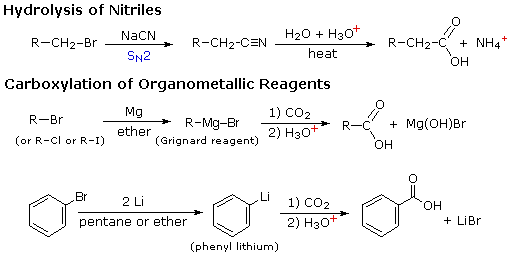
Two other useful procedures for preparing carboxylic acids involve hydrolysis of nitriles and carboxylation of organometallic intermediates. As shown in the following diagram, both methods begin with an organic halogen compound and the carboxyl group eventually replaces the halogen. Both methods require two steps, but are complementary in that the nitrile intermediate in the first procedure is generated by a SN2 reaction, in which cyanide anion is a nucleophilic precursor of the carboxyl group. The hydrolysis may be either acid or base-catalyzed, but the latter give a carboxylate salt as the initial product.
In the second procedure the electrophilic halide is first transformed into a strongly nucleophilic metal derivative, and this adds to carbon dioxide (an electrophile). The initial product is a salt of the carboxylic acid, which must then be released by treatment with strong aqueous acid.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|