


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-9-2018
Date: 18-5-2017
Date: 19-7-2019
|
The carbon-carbon bond is not completely free to rotate – there is indeed a small, 3 kcal/mol barrier to rotation that must be overcome for the bond to rotate from one staggered conformation to another. This rotational barrier is not high enough to prevent constant rotation except at extremely cold temperatures. However, at any given moment the molecule is more likely to be in a staggered conformation - one of the rotational ‘energy valleys’ - than in any other state. The potential energy associated with the various conformations of ethane varies with the dihedral angle of the bonds, as shown below.
Figure 3.6.X: The potential energy associated with the various conformations of ethane varies with the dihedral angle of the bonds.
Although the conformers of ethane are in rapid equilibrium with each other, the 3 kcal/mol energy difference leads to a substantial preponderance of staggered conformers (> 99.9%) at any given time. The animation below illustrates the relationship between ethane's potential energy and its dihedral angle
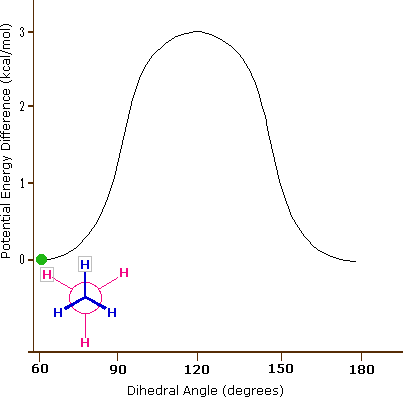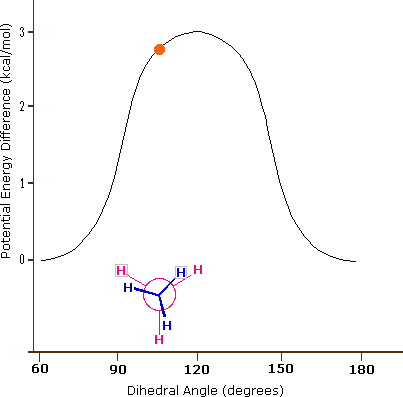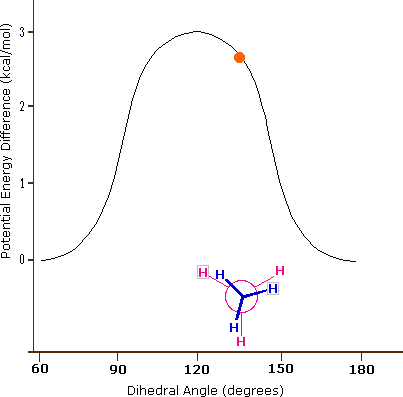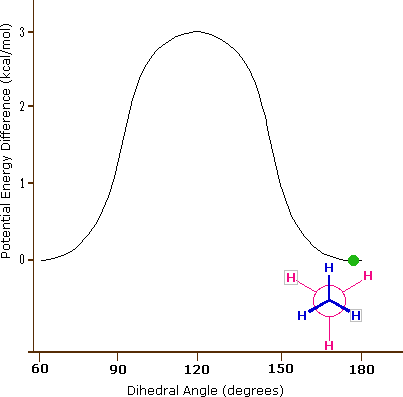
Figure 3.6.X: Animation of potential energy vs. dihedral angle in ethane



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
العتبة العباسية المقدسة تبحث مع العتبة الحسينية المقدسة التنسيق المشترك لإقامة حفل تخرج طلبة الجامعات
|
|
|